Thermodynamics and Thermochemistry 1 Question 41
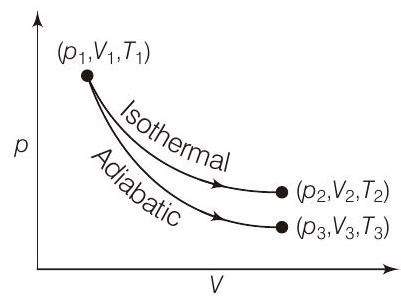
44. The reversible expansion ob an ideal gas under adiabatic and isothermal conditions is shown in the figure. Which of the following statement(s) is (are) correct?
(2012)
(a)
(b)
(c)
(d)
Show Answer
Solution:
- (a) Since, change of state
(b) Since, change of state
(c) Work done is the area under the curve of
(d)
In isothermal process,
In adiabatic process,
NOTE Here only magnitudes of work is being considered otherwise both works have negative sign.






