Thermodynamics and Thermochemistry 1 Question 39
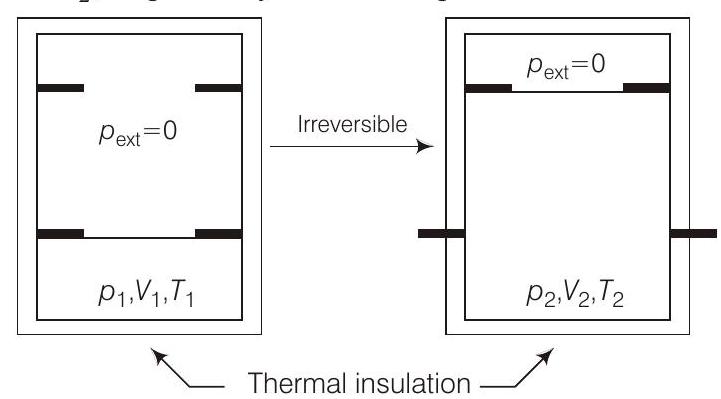
42. An ideal gas in thermally insulated vessel at internal pressure
(a)
(b)
(c)
(d)
(2014 Adv.)
Show Answer
Solution:
- PLAN This problem includes concept of isothermal adiabatic irreversible expansion.
Process is adiabatic because of the use of thermal insolution therefore,
Internal energy can be written as
The change in internal energy of an ideal gas depends only on temperature and change in internal energy
and
(d)
Hence, only (a), (b) and (c) are correct choices.






