Chapter 02 Structure of Atom
Multiple Choice Questions (MCQs)
1. Which of the following conclusions could not be derived from Rutherford’s $\alpha$-particle scattering experiment?
(a) Most of the space in the atom is empty
(b) The radius of the atom is about $10^{-10} \mathrm{~m}$ while that of nucleus is $10^{-15} \mathrm{~m}$
(c) Electrons move in a circular path of fixed energy called orbits
(d) Electrons and the nucleus are held together by electrostatic forces of attraction
Answer (c) Concept of electrons move in a circular path of fixed energy called orbits was put forward by Bohr and not derived from Rutherford’s scattering experiment. Out of a large number of circular orbits theoretically possible around the nucleus, the electron revolve only in those orbits which have a tired value of energy. Hence, these orbits are called energy level or stationary states.Show Answer
(a) $1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 3 d^{8} 4 s^{2}$
(b) $1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 3 d^{9} 4 s^{2}$
(c) $1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 3 d^{10} 4 s^{1}$
(d) $1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 3 d^{5} 4 s^{1}$
Answer (b) Correct configuration should be $1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 3 d^{10} 4 s^{1}$ for the copper which has atomic number $29\left({ }_{29} \mathrm{Cu}\right)$. Due to extra stability of full filled orbital of $d$-subshell, the last electron enter into $d$-orbital insted of $s$-orbital.Show Answer

1

2
The density of dots in a region represents the probability density of finding electrons in the region.
On the basis of above diagram which of the following statements is incorrect?
(a) $1 \mathrm{~s}$ and $2 \mathrm{~s}$ orbitals are spherical in shape
(b) The probability of finding the electron is maximum near the nucleus
(c) The probability of finding the electron at a given distance is equal in all directions
(d) The probability density of electrons for $2 s$ orbital decreases uniformly as distance from the nucleus increases
Thinking Process (i) To solve the problem, it keep in mind that s-orbital has spherical shape. (ii) Probability density represents the probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., $|\Psi|^{2}$ at that point. Answer (d) The probability density of electrons in $2 s$ orbital first increases then decreases and after that it begins to increases again as distance increases from nucleus.Show Answer
(a) They start from the cathode and move towards the anode
(b) They travel in straight line in the absence of an external electrical or magnetic field
(c) Characteristics of cathode rays do not depend upon the material of electrodes in cathode ray tube
(d) Characteristics of cathode rays depend upon the nature of gas present in the cathode ray tube
Answer (d) Cathode rays consist of negatively charged material particles called electrons. It was discovered by William Crookes. The characteristics of cathode rays do not depend upon the material of electrodes and the nature of the gas present in the cathode ray tube.Show Answer
(a) It is a negatively charged particle
(b) The mass of electron is equal to the mass of neutron
(c) It is a basic constituent of all atoms
(d) It is a constituent of cathode rays
Answer (b) The mass of electron is very small as compared to the mass of the neutron. Mass of electron $=9.1 \times 10^{-31} \mathrm{~kg}$ Mass of neutron $=1.67 \times 10^{-27} \mathrm{~kg}$Show Answer
(a) Overall neutrality of atom
(b) Spectra of hydrogen atom
(c) Position of electrons, protons and neutrons in atom
(d) Stability of atom
Answer (a) $\mathrm{J} J \mathrm{Thomson}$, in 1898, proposed plum pudding, (raisin pudding or watermelon) model of atom. An important feature of this model is that the mass of the atom is assumed to be uniformly distributed over the atom. This model was able to explain the overall neutrality of the atom.Show Answer
(a) they have same atomic number but different mass number
(b) they have same number of electrons but different number of neutrons
(c) they have same number of neutrons but different number of electrons
(d) sum of the number of protons and neutrons is same but the number of protons is different
Answer (d) Isobars have the same mass number (i.e., sum of protons and neutrons) but different atomic number (i.e., number of protons). e.g., $_{18} Ar^{40} and _{19} K^{40}$ and are isobars.Show Answer
${ }_{18} \mathbf{A r}^{\mathbf{4 0}}$
${ }_{19} \mathbf{K}^{\mathbf{4 0}}$
Atomic number $=18$
Atomic number $=19$
Mass number $=40$
Mass number $=40$
(a) 3
(b) 4
(c) 2
(d) 1
Answer (d) For a hydrogen atom wave function, there are $n-l-1$ radial nodes and $(n-1)$ total nodes. Number of radial nodes $2 n 3 p$ orbital $=n-l-1$ $$
=3-1-1=1
$$Show Answer
(a) 4
(b) 3
(c) 2
(d) 1
Answer (c) Number of angular nodes $=l$ For $4^{\text {th }}$ orbital $(n=4)$ and $l=2$ for $d$-orbital $\therefore$ Number of angular nodes $=2$Show Answer
(a) Pauli’s exclusion principle
(b) Heisenberg’s uncertainty principle
(c) Hund’s rule of maximum multiplicity
(d) Aufbau principle
Answer (b) Werner Heisenberg, a German physicist in 1927, stated uncertainty principle which states that it is impossible to determine simultaneously, the exact position and exact momentum of an electron. Mathematically, $$
\Delta x \times \Delta p \geq \frac{h}{4 \pi}
$$ The important implications of the Heisenberg uncertainty principle is that it rules out existence of definite paths or trajectories of electrons and other similar particles.Show Answer
(a) 2
(b) 4
(c) 9
(d) 3
Answer (c) Total number of orbitals associated with $n^{\text {th }}$ shell $=n^{2}$ $\therefore$ Total number of orbitals associated with third shell $=(3)^{2}=9$Show Answer
(a) $l$
(b) $n$ and $l$
(c) $n$ and $m$
(d) $m$ and $s$
Answer (a) Orbital angular momentum $m v r=\frac{h}{2 \pi} \sqrt{l(l+1)}$. Hence, it depends only on ’ $l$ ‘. $l$ can have values ranging from 0 to $(n-1)$. (a) When $l=0$, the subshell is $s$ and orbital is spherical in shape. (b) When $l=1$, the subshell is $p$ and orbital is dumb-bell shaped. (c) When $l=2$, the subshell is $d$ and orbital is double dumb-bell shaped. (d) When $l=3$, the subshell is $f$ and orbital is complicated in shape.Show Answer
(a) $1: 2$
(b) $1: 1$
(c) $1: 3$
(d) $3: 1$
Answer (c) The fractional atomic mass ( 35.5 ) of chlorine is due to the fact that in ordinary chlorine atom, $\mathrm{Cl}-37$ and $\mathrm{Cl}-35$ are present in the ratio of $1: 3$. $\therefore$ Average atomic mass of $\mathrm{Cl}=\frac{3 \times 35+1 \times 37}{4}=35.5 \mathrm{amu}$Show Answer
(a) $\mathrm{Cr}^{3+}, \mathrm{Fe}^{3+}$
(b) $\mathrm{Fe}^{3+}, \mathrm{Mn}^{2+}+$
(c) $\mathrm{Fe}^{3+}, \mathrm{Co}^{3+}$
(d) $\mathrm{Sc}^{3+}, \mathrm{Cr}^{3+}$
Answer (b)
$ _{24} Cr=[Ar] 3 d^{5}, 4 s^{1}\quad \quad$
$ _{24} Cr^{3+} =[Ar] 3 d^{3} $ $ _{26} Fe^{3+} =[Ar] 3 d^{5} \quad \quad $
$ _{26} Fe =[Ar] 3 d^{6}, 4 s^{2} $ $ _{25} Mn^{2+} =[Ar] 3 d^{5} \quad \quad$
$ _{25} Mn =[Ar] 3 d^{5}, 4 s^{2} $ $ _{27} Co^{3+} =[Ar] 3 d^{6} \quad \quad$
$ _{27} Co =[Ar] 3 d^{7}, 4 s^{2} $ $ _{21} Sc^{3+} =[Ar] \quad \quad $
$ _{21} Sc =[Ar] 3 d^{1}, 4 s^{2}$ Thus, $ Fe^{3+}$ and $ Mn^{2+}$ have the same electronic configuration.Show Answer
(a) $Z_{\text {eff }}$ for an electron in a $2 s$ orbital is the same as $Z_{\text {eff }}$ for an electron in a $2 p$ orbital
(b) An electron in the $2 s$ orbital has the same energy as an electron in the $2 p$ orbital
(c) $Z_{\text {eff }}$ for an electron in $1 s$ orbital is the same as $Z_{\text {eff }}$ for an electron in a $2 s$ orbital
(d) The two electrons present in the $2 s$ orbital have spin quantum numbers $m_{s}$ but of opposite sign
Answer (d) (a) Electrons in $2 s$ and $2 p$ orbitals have different screen effect. Hence, their $Z_{\text {eff }}$ is different. $Z_{\text {eff }}$ of $2 s$ orbital $>Z_{\text {eff }}$ of $2 p$ orbital Therefore, it is not correct. (b) Energy of $2 s$ orbital $<$ energy of $2 p$ orbital. Hence, it is not correct. (c) $Z_{\text {eff }}$ of 1 sorbital $\neq Z_{\text {eff }}$ of 2 s orbital Hence, it is incorrect. (d) For the two electrons of $2 s$ orbital, the value of $m_{s}$ is $+\frac{1}{2}$ and $-\frac{1}{2}$. Hence, it is correct.Show Answer
(a) Electron
(b) Alpha particle $\left(\mathrm{He}^{2+}\right)$
(c) Neutron
(d) Proton
Answer (b) From de-Broglie equation, $$
\text { wavelength, } \lambda=\frac{h}{m v}
$$ For same speed of different particles, i.e., electron, proton, neutron and $\alpha$-particle, $$
\lambda \propto \frac{1}{m}
$$ As $h$ is constant. Greater the mass of matter waves, lesser is wavelength and vice-versa. In these matter waves, alpha particle $\left(\mathrm{He}^{2+}\right)$ has higher mass, therefore, shortest wavelength.Show Answer
17. Identify the pairs which are not of isotopes?
(a) $ _{6}^{12} X, _{6}^{13} Y$
(b) $ _{17}^{35} X, _{17}^{37} Y$
(c) $ _{6}^{14} X, _{7}^{14} Y$
(d) $ _{4}^{8} X, _{5}^{8} Y$
Thinking Process (i) Isotopes are the elements that have same atomic number $(Z)$ but different mass number (A). (ii) The digit written as subscript represents the atomic number $(Z)$ and that as superscript represents the mass number (A) of the element. Answer (c, $d$) (a) $ _{6}^{12} X$ and $ _{6}^{13} Y$ have same atomic number but different mass number. (b) $ _{17}^{35} X$ and $ _{17}^{37} Y$ have same atomic number but different mass number. Both these pairs are isotopes to each other. (c) $ _{6}^{14} X$ and $ _{7}^{14} Y$ have different atomic number but same mass number. (d) $ _{4}^{8} X$ and $ _{5}^{8} Y$ have different atomic number but same mass number. Both these pairs are isobars to each other.Show Answer
(a) (i) $n=3, l=2, m_{l}=-2, m_{s}=-\frac{1}{2}$ (ii) $n=3, l=2, m_{l}=-1, m_{s}=-\frac{1}{2}$
(b) (i) $n=3, l=1, m_{l}=1, m_{s}=+\frac{1}{2}$ (ii) $n=3, l=2, m_{l}=1, m_{s}=+\frac{1}{2}$
(c) (i) $n=4, l=1, m_{l}=1, m_{s}=+\frac{1}{2}$ (ii) $n=3, l=2, m_{l}=1, m_{s}=+\frac{1}{2}$
(d) (i) $n=3, l=2, m_{l}=+2, m_{s}=-\frac{1}{2}$ (ii) $n=3, l=2, m_{l}=+2, m_{s}=+\frac{1}{2}$
Answer $(a, d)$ Degenerate orbitals means the orbitals of the same subshell of the same main shell, i.e., their $n$ and $l$ value. (a) (i) $3 d_{x y} \quad$
(ii) $3 d_{y z}$ (b) (i) $3 p_{x} \quad$
(ii) $3 d_{x y}$ (c) (i) $4 s \quad$
(ii) $3 d_{x y}$ (d) (i) $3 d_{x^{2}-y^{2}} \quad$
(ii) $3 d_{x^{2}-y^{2}}$ Thus, $3 d_{x y}$ and $3 d_{y z} ; 3 d_{x^{2}-y^{2}}$ and $3 d_{x^{2}-y^{2}}$ represent pair of degenerate orbitals.Show Answer
| $n$ | $l$ | $m$ | $n$ | $l$ | $m$ |
|---|---|---|---|---|---|
| (a) 1 | 1 | +2 | (b) 2 | 1 | +1 |
| (c) 3 | 2 | -2 | (d) 3 | 4 | -2 |
Answer ( $b, c)$Show Answer
If $\quad n=1, l \neq 1(\because l<n)$
$(\because l<n)$.
Hence, (a) is incorrect.
If $\quad n=2, l=0,1$, For $l=1, m=-1,0,+1$.
Hence, (b) is correct.
If $\quad n=3, l=0,1,2$
For $\quad l=2, m=-2,-1,0,+1,+2$.
$(\because l<n)$.
Hence, (c) is correct.
If $\quad n=3, l \neq 4$
Hence, (d) is incorrect.
(a) $\mathrm{Na}^{+}, \mathrm{Mg}^{2+}$
(b) $\mathrm{AP}^{3+}, \mathrm{O}^{-}$
(c) $\mathrm{Na}^{+}, \mathrm{O}^{2-}$
(d) $\mathrm{N}^{3-}, \mathrm{Cl}^{-}$
Thinking Process To solve this problem, it should kept in mind that isoelectronic are those species which have same number of electrons. Answer $(a, c)$ For, $$
\begin{aligned}
\mathrm{Na}^{+} & =11-1=10 e^{-} \\
\mathrm{Mg}^{2+} & =12-2=10 e^{-}
\end{aligned}
$$ Thus, they have same number of electrons. For, $$
\mathrm{Al}^{3+}=13-3=10 e^{-}, \mathrm{O}^{-}=8+1=9 e^{-} .
$$ They do not have same number of electrons. For, $$
\mathrm{Na}^{+}=10 e^{-}, \mathrm{O}^{2-}=8+2=10 e^{-}
$$ They have same number of electrons. $$
\text { For, } \quad \mathrm{N}^{3-}=7+3=10 e^{-}, \mathrm{Cl}^{-}=17+1=18 e^{-} .
$$ They do not have same number of electrons. Thus, $\mathrm{Na}^{+}$is isoelectronic with $\mathrm{Mg}^{2+}$ and $\mathrm{O}^{2-}$.Show Answer
(a) Angular quantum number determines the three dimensional shape of the orbital
(b) The principal quantum number determines the orientation and energy of the orbital
(c) Magnetic quantum number determines the size of the orbital
(d) Spin quantum number of an electron determines the orientation of the spin of electron relative to the chosen axis
Show Answer
Answer
$(a, d)$
(a) Azimuthal quantum number ’ $l$ ’ is also known as orbital angular momentum or subsidiary quantum number. It determines three dimensional shape of the orbital.
(b) The principal quantum number determines the size of the orbit.
(c) Magnetic quantum number determines the orientation of the electron cloud in a subshell.
(d) An electron spins around its own axis, much in a similar way as earth spins around its own axis while revolving around the sun. In other words, an electron has, besides charge and mass, intrinsic spin angular quantum number.
Short Answer Type Questions
22. Arrange $s, p$ and $d$ subshells of a shell in the increasing order of effective nuclear charge ( $Z_{\text {eff }}$ ) experienced by the electron present in them.
Answer s-orbital is spherical in shape, it shields the electrons from the nucleus more effectively than $p$ - orbital which in turn shields more effectively than $d$-orbital. Therefore, the effective nuclear charge $\left(Z_{\text {eff }}\right)$ experienced by electrons present in them is $d<p<s$.Show Answer
Answer From the orbital diagram, it is seen that there are two unpaired electrons.Show Answer

Answer ${ }_{28} \mathrm{Ni}=1 s^{2}, 2 s^{2}, 2 p^{6}, 3 s^{2}, 3 p^{6}, 3 d^{8}, 4 s^{2}$; Nickel will lose 2 electrons from $4 s$ (outermost shell) to form $\mathrm{Ni}^{2+}$ ion. Hence, $$
{ }_{28} \mathrm{Ni}^{2+}=1 s^{2}, 2 s^{2}, 2 p^{6}, 3 s^{2}, 3 p^{6}, 3 d^{8}, 4 s^{0} .
$$Show Answer
$$ 3 d_{x y}, 4 d_{x y}, 3 d_{z^{2}}, 3 d_{y z}, 4 d_{y z}, 4 d_{z^{2}} $$
Answer The orbitals which belongs to same subshell and same shell are called degenerate orbitals. $\left(3 d_{x y}, 3 d_{z^{2}}, 3 d_{y z}\right)$ and $\left(4 d_{x y}, 4 d_{y z}, 4 d_{z^{2}}\right)$ are the two sets of degenerate orbitals.Show Answer
Thinking Process To calculate the total number and radial nodes of principal quantum number $n$, the following formula is used (i) Radial nodes (or spherical nodes) $=n-l-1$ (ii) Angular nodes (or non-spherical nodes) $=l$ (iii) Total nodes $=n-1$ Answer For $3 p$-orbital, principal quantum number, $n=3$ and azimuthal quantum number $l=1$ Number of angular nodes $=l=1$ Number of radial nodes $=n-l-1=3-1-1=1$Show Answer
I. Based upon the above information, arrange the following orbitals in the increasing order of energy.
(a) $1 s, 2 s, 3 s, 2 p$
(b) $4 s, 3 s, 3 p, 4 d$
(c) $5 p, 4 d, 5 d, 4 f, 6 s$
(d) $5 f, 6 d, 7 s, 7 p$
II. Based upon the above information. Solve the questions given below.
(a) Which of the following orbitals has the lowest energy?
$$ 4 d, 4 f, 5 s, 5 p $$
(b) Which of the following orbitals has the highest energy?
$$ 5 p, 5 d, 5 f, 6 s, 6 p $$
Answer I.(a) $(n+l)$ values of $1 s=1+0=1,2 s=2+0=2,3 s=3+0=3,2 p=2+1=3$ Hence, increasing order of their energy is $$
1 s<2 s<2 p<3 s
$$ (b) $4 s=4+0=4,3 s=3+0=3,3 p=3+1=4,4 d=4+2=6$ Hence, $$
3 s<3 p<4 s<4 d
$$ (c) $5 p=5+1=6,4 d=4+2=6,5 d=5+2=7,4 f=4+3=7,6 s=6+0=6$ Hence, $\quad 4 d<5 p<6 s<4 f<5 d$ (d) $5 f=5+3=8,6 d=6+2=8,7 s=7+0=7,7 p=7+1=8$. Hence, $\quad 7 s<5 f<6 d<7 p$ II. (a) $(n+l)$ values of $4 d=4+2=6,4 f=4+3=7,5 s=5+0=5,7 p=7+1=8$ Hence, $5 s$ has the lowest energy. (b) $5 p=5+1=6,5 d=5+2=7,5 f=5+3=8,6 s=6+0=6,6 p=6+1=7$ Hence, $5 f$ has highest energy.Show Answer
Proton, cathode rays, electron, neutron.
Answer Neutron being neutral will not show deflection from the path on passing through an electric field. Proton, cathode rays and electron being the charged particle will show deflection from the path on passing through an electric field.Show Answer
Thinking Process (i) Atomic mass $(A)=$ number of neutron $(n)+$ number of proton $(p)$ (ii) Number of proton is equal to the atomic number of the atom. Answer An atom having atomic mass number 13 and number of neutrons 7 . $$
\begin{aligned}
& i.e., \quad A=13, n=7 \\
& \text{ As we know that},\quad A=n+p \\
& \therefore \quad p=A-n=13-7=6 \\
& Hence, \quad Z=p=6
\end{aligned}
$$Show Answer
$\lambda(\mathrm{A})=300 \mathrm{~nm} \lambda$
(B) $=300 \mu \mathrm{m} \lambda$
$(\mathrm{C})=3 \mathrm{~nm} \lambda$
(D) $=30 \AA$
Arrange these radiations in the increasing order of their energies.
Answer $\lambda(A)=300 \mathrm{~nm}=300 \times 10^{-9} \mathrm{~m}, $ $ \lambda(B)=300 \mu \mathrm{m}=300 \times 10^{-6} \mathrm{~m}$ $\lambda(\mathrm{C})=3 \mathrm{~nm}=3 \times 10^{-9} \mathrm{~m}$, $\lambda(\mathrm{D})=30 \AA=30 \times 10^{-10} \mathrm{~m}=3 \times 10^{-9} \mathrm{~m}$ $Energy, E=\frac{h c}{\lambda}$ $ Therefore,\quad E \propto \frac{1}{\lambda}$ Increasing order of energy is $B<A<C=D$Show Answer
Answer Configurations either exactly half-filled or fully filled orbitals are more stable due to symmetrical distribution of electrons and maximum exchange energy. In $3 d^{10} 4 s^{1}, d$-orbitals are completely filled and s-orbital is half-filled. Hence, it is more stable configuration.Show Answer
$\left(R_{\mathrm{H}}=109677 \mathrm{~cm}^{-1}\right)$
Answer From Rydberg formula, Wave number, $\quad \bar{v}=109677\left[\frac{1}{n_{i}^{2}}-\frac{1}{n_{f}^{2}}\right] \mathrm{cm}^{-1}$ $$
\begin{aligned}
Given, \quad n_{i} & =2 \text { and } n_{f}=4 \quad \text{(Transition in Balmer series)}\\
\bar{v} & =109677\left[\frac{1}{2^{2}}-\frac{1}{4^{2}}\right] \mathrm{cm}^{-1}
\end{aligned}
$$ $$
\begin{array}{ll}
\Rightarrow & \bar{v}=109677\left[\frac{1}{4}-\frac{1}{16}\right] \mathrm{cm}^{-1} \\
\Rightarrow & \bar{v}=109677 \times\left[\frac{4-1}{16}\right] \mathrm{cm}^{-1} \\
\Rightarrow & \bar{v}=20564.44 \mathrm{~cm}^{-1}
\end{array}
$$Show Answer
Answer Given, $$
\begin{aligned}
& m=100 \mathrm{~g}=0.1 \mathrm{~kg} \\
& v=100 \mathrm{~km} / \mathrm{h}=\frac{100 \times 1000}{60 \times 60}=\frac{1000}{36} \mathrm{~ms}^{-1}
\end{aligned}
$$ From de-Broglie equation, wavelength, $\lambda=\frac{h}{m v}$ $$
\lambda=\frac{6.626 \times 10^{-34} \mathrm{~kg} \mathrm{~m}^{2} \mathrm{~s}^{-1}}{0.1 \mathrm{~kg} \times \frac{1000}{36} \mathrm{~ms}^{-1}}=238.5 \times 10^{-36} \mathrm{~m}
$$ As the wavelength is very small so wave nature cannot be detected.Show Answer
Answer The line spectrum of any element has lines corresponding to definite wavelengths. Lines are obtained as a result of electronic transitions between the energy levels. Hence, the electrons in these levels have fixed energy, i.e., quantized values.Show Answer
Answer From de-Broglie equation, wavelength, $\lambda=\frac{h}{m v}$ For same wavelength for two different particles, i.e., electron and proton, $m_{1} v_{1}=m_{2} v_{2}(h$ is constant). Lesser the mass of the particle, greater will be the velocity. Hence, electron will have higher velocity.Show Answer
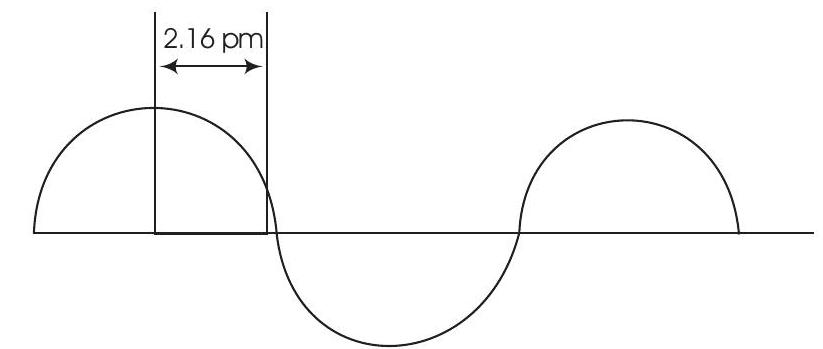
Answer Wavelength is the distance between two successive peaks or two successive throughs of a wave. Therefore, $$
\begin{aligned}
\lambda=4 \times 2.16 \mathrm{pm} & =8.64 \mathrm{pm} \\
& =8.64 \times 10^{-12} \mathrm{~m} \quad\left[\because 1 \mathrm{pm}=10^{-12} \mathrm{~m}\right]
\end{aligned}
$$Show Answer
Which part of the electromagnetic spectrum does it belong to?
Answer $$
Wavelength, \quad\lambda=\frac{C}{V}
$$ $$
\begin{aligned}
Given, \quad \text { frequency } v & =4.620 \times 10^{14} \mathrm{~Hz} \text { or } 4.620 \times 10^{14} \mathrm{~s}^{-1} \\
\lambda & =\frac{c}{v}=\frac{3 \times 10^{8} \mathrm{~ms}^{-1}}{4.620 \times 10^{14} \mathrm{~s}^{-1}} \\
& =0.6494 \times 10^{-6} \mathrm{~m} \\
& =649.4 \mathrm{~nm} \quad \quad\left[\because 1 \mathrm{~nm}=10^{-6} \mathrm{~m}\right]
\end{aligned}
$$ Thus, it belongs to visible region.Show Answer
Answer Difference between the terms orbit and orbital are as belowShow Answer
Orbit
Orbital
An orbit is a well defined circular path
around the nucleus in which the electrons
revolve.An orbital is the three dimensional space
around the nucleus within which the
probability of finding an electron is
maximum (upto 90%).
All orbits are circular and disc like.
The concept of an orbit is not in accordance orbitals have different shapes.
with the wave character of electrons withThe concept of an orbital is in accordance
with the wave character of electrons and
uncertainty principle.
The maximum number of electrons in any
orbit is given by $2 n^{2}$ where $n$ is the number
of the orbit.The maximum number of electrons present
in any orbital is two.
Answer Given that, speed $=90 \mathrm{~m} / \mathrm{s}$ mass $=10 \mathrm{~g}=10 \times 10^{-3} \mathrm{~kg}$ Uncertainty in speed $(\Delta v)=4 $% of $90 \mathrm{~ms}^{-1}=\frac{4 \times 90}{100}=3.6 \mathrm{~ms}^{-1}$ From Heisenberg uncertainty principle, $$
\begin{aligned}
\Delta x \cdot \Delta v & =\frac{h}{4 \pi m} \\
or \quad \Delta x & =\frac{h}{4 \pi m \Delta v}
\end{aligned}
$$ Uncertainty in position, $$
\begin{aligned}
\Delta x & =\frac{6.626 \times 10^{-34} \mathrm{kgm}^{2} \mathrm{~s}^{-1}}{4 \times 3.14 \times 10 \times 10^{-3} \mathrm{~kg} \times 3.6 \mathrm{~ms}^{-1}} \\
& =1.46 \times 10^{-33} \mathrm{~m}
\end{aligned}
$$Show Answer
Answer If uncertainty principle is applied to an object of mass, say about a milligram $\left(10^{-6} \mathrm{~kg}\right)$, then $$
\begin{aligned}
\Delta \cdot \Delta x & =\frac{h}{4 \pi m} \\
\Delta v \cdot \Delta x & =\frac{6.626 \times 10^{-34} \mathrm{kgm}^{2} \mathrm{~s}^{-1}}{4 \times 3.14 \times 10^{-6} \mathrm{~kg}} \\
& =0.52 \times 10^{-28} \mathrm{~m}^{2} \mathrm{~s}^{-1}
\end{aligned}
$$ The value of $\Delta v \cdot \Delta x$ obtained is extremely small and is insignificant. Therefore, for milligram-sized or heavier objects, the associated uncertainties are hardly of any real consequence.Show Answer
Show Answer
Answer
In hydrogen atom, the energy of an electron is determined by the value of $n$ and in multielectron atom, it is determined by $n+l$. Hence, for a given principal quantum, number electrons of $s, p, d$ and $f$-orbitals have different energy (for $s, p, d$ and $f, l=0,1,2$ and 3 respectively).
Matching the Columns
42. Match the following species with their corresponding ground state electronic configuration.
| Atom / Ion | Electronic configuration | ||
|---|---|---|---|
| A. | $\mathrm{Cu}$ | 1. | $1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 3 d^{10}$ |
| B. | $\mathrm{Cu}^{2+}$ | 2. | $1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 3 d^{10} 4 s^{2}$ |
| C. | $\mathrm{Zn}^{2+}$ | 3. | $1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 3 d^{10} 4 s^{1}$ |
| D. | $\mathrm{Cr}^{3+}$ | 4. | $1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 3 d^{9}$ |
| 5. | $1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 3 d^{3}$ |
Answer A. $\rightarrow(3)$ B. $\rightarrow(4)$ C. $\rightarrow(1)$ D. $\rightarrow(5)$ A. $\mathrm{Cu}(Z=29) \quad: \quad 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 3 d^{10} 4 s^{1}$ B. $\mathrm{Cu}^{2+}(Z=29): 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 3 d^{9}$ C. $\mathrm{Zn}^{2+}(Z=30): 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 3 d^{10}$ D. $\mathrm{Cr}^{3+}(Z=24): 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 3 d^{3}$Show Answer
| Quantum number | Information provided | ||
|---|---|---|---|
| A. | Principal quantum number | 1. | Orientation of the orbital |
| B. | Azimuthal quantum number | 2. | Energy and size of orbital |
| C. | Magnetic quantum number | 3. | Spin of electron |
| D. | Spin quantum number | 4. | Shape of the orbital |
Answer A. $\rightarrow(2)$ B. $\rightarrow(4)$ C. $\rightarrow(1)$ D. $\rightarrow(3)$ A. Principal quantum number is the most important quantum number as it determines the size and to large extent the energy of the orbital. B. Azimuthal quantum number determines the angular momentum of the electron and defines the three-dimensional shape of the orbital. C. Magnetic quantum number gives information about the spatial orientation of orbitals with respect to a standard set of coordinate axes. D. Spin quantum number arises from the spectral evidence that an electron in its motion around the nucleus in an orbit also rotates or spin about its own axis.Show Answer
| Rules | Statements | |
|---|---|---|
| Hund’s Rule | 1. No two electrons in an atom can have the same set of four quantum numbers. |
|
| B. | Aufbau Principle | 2. Half-filled and completely filled orbitals have extra stability. |
| C. | Pauli Exclusion Principle | 3. Pairing of electrons in the orbitals belonging to the same subshell does not take place until each orbital is singly occupied. |
| D. | Heisenberg’s Uncertainty Principle |
4. It is impossible to determine the exact position and exact momentum of a subatomic particle simultaneously. 5. In the ground state of atoms, orbitals are filled in the order of their increasing energies. |
Answer A. $\rightarrow(3)$ B. $\rightarrow$ (5) C. $\rightarrow(1)$ D. $\rightarrow(4)$ A. Hund’s rule states that pairing of electrons in the orbitals belonging to the same subshell ( $p, d$ or $f$ ) does not take place until each orbital belonging to that subshell has got one electron each i.e., it is singly occupied. B. Aufbau principle states that in the ground state of the atoms, the orbitals are filled in order of their increasing energies. C. According to Pauli exclusion principle, no two electrons in an atom can have the same set of four quantum numbers. D. Heisenberg’s uncertainty principle states that it is impossible to determine the exact position and exact momentum of a subatomic particle simultaneously.Show Answer
| Column I | Column II | ||
|---|---|---|---|
| A. | X-rays | 1. | $v=10^{0}-10^{4} \mathrm{~Hz}$ |
| B. | Ultraviolet wave (UV) | 2. | $v=10^{10} \mathrm{~Hz}$ |
| C. | Long radio waves | 3. | $v=10^{16} \mathrm{~Hz}$ |
| D. | Microwave | 4. | $v=10^{18} \mathrm{~Hz}$ |
Answer A. $\rightarrow(4)$ B. $\rightarrow(3)$ C. $\rightarrow$ (1) D. $\rightarrow(2)$Show Answer
Name
Frequency
Uses
A.
X-rays
$2 \times 10^{16}-3 \times 10^{19} \mathrm{~Hz}$
Medical pictures, material testing
B.
Ultraviolet wave $(\mathrm{UV})$
$7.9 \times 10^{14}-2 \times 10^{16} \mathrm{~Hz}$
Germisidal lamp
C.
Long radio waves
$10^{0}-10^{4} \mathrm{~Hz}$
Signal transmission
D.
Microwave
$1 \times 10^{9}-5 \times 10^{11} \mathrm{~Hz}$
Cooking radar
| Column I | Column II | ||
|---|---|---|---|
| A. | Photon | 1. | Value is 4 for $N$-shell |
| B. | Electron | 2. | Probability density |
| C. | $\psi^{2}$ | 3. | Always positive value |
| D. | Principal quantum number $n$ |
4. | Exhibits both momentum and wavelength |
Answer A. $\rightarrow(4)$ B. $\rightarrow(4)$ C. $\rightarrow(2,3)$ D. $\rightarrow(1,3)$ A. Photon has particle nature as well as wave nature. It exhibits both momentum and wavelength. B. Electron also has particle nature as well as wave nature. Thus, it also exhibits both momentum and wavelength. C. $\psi^{2}$ represents probability density of electron and always has positive values. D. Principal quantum number $n=4$ for $N$-shell. $$
\begin{aligned}
& K\llcorner M N \\
& n=1234
\end{aligned}
$$ It always has positive values.Show Answer
| Column I | Column II | ||
|---|---|---|---|
| A. | $\mathrm{Cr}$ | 1. | $[\mathrm{Ar}] 3 d^{8} 4 s^{0}$ |
| B. | $\mathrm{Fe}^{2+}$ | 2. | $[\mathrm{Ar}] 3 d^{10} 4 s^{1}$ |
| C. | $\mathrm{Ni}^{2+}$ | 3. | $[\mathrm{Ar}] 3 d^{6} 4 s^{0}$ |
| D. | $\mathrm{Cu}$ | 4. | $[\mathrm{Ar}] 3 d^{5} 4 s^{1}$ |
| 5. | $[\mathrm{Ar}] 3 d^{6} 4 s^{2}$ |
Answer A. $\rightarrow(4)$ B. $\rightarrow(3)$ C. $\rightarrow(1)$ D. $\rightarrow(2)$ A. $\operatorname{Cr}(Z=24)=1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 3 d^{5} 4 s^{1}=[\operatorname{Ar}] 3 d^{5} 4 s^{1}$ B. $\mathrm{Fe}^{2+}(Z=26)=1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 3 d^{6} 4 s^{0}=[\operatorname{Ar}] 3 d^{6} 4 s^{0}$ C. $\mathrm{Ni}^{2+}(Z=28)=1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 3 d^{8} 4 s^{0}=[\operatorname{Ar}] 3 d^{8} 4 s^{0}$ D. $\mathrm{Cu}(Z=29)=1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 3 d^{10} 4 s^{1}=[\mathrm{Ar}] 3 d^{10} 4 s^{1}$ In the following questions a statement of Assertion (A) followed by a statement of Reason $(\mathrm{R})$ is given. Choose the correct option out of the choices given below in each question.Show Answer
Assertion and Reason
Reason (R) The chemical properties of an atom are controlled by the number of electrons in the atom.
(a) Both $A$ and $R$ are true and $R$ is the correct explanation of $A$
(b) Both $A$ and $R$ are true but $R$ is not the correct explanation of $A$
(c) $A$ is true but $R$ is false
(d) Both $A$ and $R$ are false
Answer (a) Both assertion and reason are true and reason is the correct explanation of assertion. Isotopes have the same atomic number i.e., same number of electrons which are responsible for their chemical behaviour. Hence, these exhibit similar chemical properties.Show Answer
Reason (R) The frequency of radiation emitted by a body goes from a lower frequency to higher frequency with an increase in temperature.
(a) Both $A$ and $R$ are true and $R$ is the correct explanation of $A$
(b) Both $A$ and $R$ are true but $R$ is not the correct explanation of $A$
(c) $A$ is true but $R$ is false
(d) Both $A$ and $R$ are false
Answer (c) Assertion is true and reason is false. A body which absorbs and emits all radiations falling on it is called perfect black body. With rise in temperature, frequency increases.Show Answer
Reason (R) The path of an electron in an atom is clearly defined.
(a) Both $A$ and $R$ are true and $R$ is the correct explanation of $A$
(b) Both $A$ and $R$ are true but $R$ is not the correct explanation of $A$
(c) $A$ is true and $R$ is false
(d) Both $A$ and $R$ are false
Show Answer
Answer
(c) Assertion is true and reason is false.
According to Heisenberg’s uncertainty principle, the exact position and exact momentum of an electron cannot be determined simultaneously. Thus, the path of electron in an atom is not clearly defined.
Long Answer Type Questions
51. What is photoelectric effect? State the result of photoelectric effect experiment that could not be explained on the basis of laws of classical physics. Explain this effect on the basis of quantum theory of electromagnetic radiations.
Answer Photoelectric effect When radiation with certain minimum frequency $\left(v_{0}\right)$ strike the surface of a metal, the electrons are ejected from the surface of the metal. This phenomenon is called photoelectric effect. The electrons emitted are called photoelectrons. Equipment for studying the photoelectric effect. Light of a particular frequency strikes a clean metal surface inside a vacuum chamber. Electrons are ejected from the metal and are counted by a detector that measures their kinetic energy. The result observed in this experiment were (i) The electrons are ejected from the metal surface as soon as the beam of light strikes the surface, i.e., there is no time lag between the striking of light beam and the ejection of electrons from the metal surface. (ii) The number of electrons ejected is proportional to the intensity or brightness of light. (iii) For each metal, there is a characteristic minimum frequency, $v_{0}$ (also known as threshold frequency) below which photoelectric effect is not observed. At a frequency $v>v_{0}$, the ejected electrons come out with certain kinetic energy. The kinetic energies of these electrons increases with the increase of frequency of the light used. The above observation cannot be explained by the electromagnetic wave theory. According to this theory, since radiations were continuous, therefore, it should be possible to accumulate energy on the surface of the metal, irrespective of its frequency and thus, radiations of all frequencies should be able to eject electrons. Similarly, according to this theory, the energy of the electrons ejected should depend upon the intensity of the incident radiation. ###Particle Nature of Electromagnetic Radiation To explain the phenomena of ‘black body radiation’ and ‘photoelectric effect’, Max Planck in 1900 , put forward a theory known after his name as Planck’s quantum theory. This theory was further extended by Einstein in 1905. The important points of this theory are as follows (i) The radiant energy is emitted or absorbed not continuously but discontinuously in the form of small discrete packets of energy. Each such packet of energy is called a ‘quantum’. In case of light, the quantum of energy is called a ‘photon’. (ii) The energy of each quantum is directly proportional to the frequency of the radiation, i.e., $E \propto v$ or $E=h v$ where, $h$ is a proportionality constant, called Planck’s constant. Its value is approximately equal to $6.626 \times 10^{-27} \mathrm{erg} \mathrm{s}$ or $6.626 \times 10^{-34} \mathrm{~J} \mathrm{~s}$. (iii) The total amount of energy emitted or absorbed by a body will be some whole number quanta. Hence, $E=n h v$ (where, $n$ is any integer). Note The energy possessed by one mole of quanta (or photons), i.e., Avogadro’s number $\left(N_{0}\right)$ of quanta is called one Einstein of energy, i.e., 1 Einstein of energy $(E)=N_{0} h v=N_{0} h \frac{c}{\lambda}$Show Answer

Show that an electron will not be emitted if a photon with a wavelength equal to $600 \mathrm{~nm}$ hits the metal surface.
Answer We know that, $$
\begin{aligned}
h v & =h v_{0}+\mathrm{KE} \\
or \quad h v-\mathrm{KE} & =h v_{0}=\left(6.626 \times 10^{-34} \mathrm{Js} \times 1 \times 10^{15} \mathrm{~s}^{-1}\right)-1.988 \times 10^{-19} \mathrm{~J} \\
h v_{0} & =6.626 \times 10^{-19}-1.988 \times 10^{-19} \mathrm{~J} \\
h v_{0} & =4.638 \times 10^{-19} \mathrm{~J} \\
v_{0} & =\frac{4.638 \times 10^{-19} \mathrm{~J}}{6.626 \times 10^{-34} \mathrm{Js}}=0.699 \times 10^{15} \mathrm{~s}^{-1} \\
When, \quad \lambda & =600 \mathrm{~nm}=600 \times 10^{-19} \mathrm{~m} \\
v & =\frac{c}{\lambda}=\frac{3.0 \times 10^{8} \mathrm{~ms}^{-1}}{6.0 \times 10^{-7} \mathrm{~m}}=0.5 \times 10^{15} \mathrm{~s}^{-1}
\end{aligned}
$$ Thus, $v<v_{0}$. hence, no electron will be emitted.Show Answer
$$ \bar{v}=109677\left[\frac{1}{n_{i}^{2}}-\frac{1}{n_{f}^{2}}\right] $$
What points of Bohr’s model of an atom can be used to arrive at this formula? Based on these points derive the above formula giving description of each step and each term.
Answer The two important points of Bohr’s model that can be used to derive the given formula are as follows (i) Electrons revolve around the nucleus in a circular path of fixed radius and energy. These paths are called orbits. stationarv states or allowed enerav states (ii) Energy is emitted or absorbed when an electron moves from higher stationary state to lower stationary state or from lower stationary state to higher stationary state respectively. Derivation The energy of the electron in the $n^{\text {th }}$ stationary state is given by the expression, $$
E_{n}=-R_{H}\left(\frac{1}{n^{2}}\right) \quad n=1,2,3 \quad …(i)
$$ where, $R_{\mathrm{H}}$ is called Rydberg constant and its value is $2.18 \times 10^{-18} \mathrm{~J}$. The energy of the lowest state, also called the ground state, is $$
E_{n}=-2.18 \times 10^{-18}\left(\frac{1}{1^{2}}\right)=-2.18 \times 10^{-18} \mathrm{~J} \quad …(ii)
$$ The energy gap between the two orbits is given by the equation, $$
\Delta E=E_{f}-E_{i} \quad …(iii)
$$ On combining Eqs. (i) and (iii) $$
\Delta E=\left(-\frac{R_{H}}{n_{f}^{2}}\right)-\left(-\frac{R_{H}}{n_{i}^{2}}\right)
$$ Where, $n_{i}$ and $n_{f}$ stand for initial orbit and final orbit. $$
\Delta E=R_{\mathrm{H}}\left[\frac{1}{n_{i}^{2}}-\frac{1}{n_{f}^{2}}\right]=2.18 \times 10^{-18} \mathrm{~J}\left[\frac{1}{n_{i}^{2}}-\frac{1}{n_{f}^{2}}\right]
$$ Frequency, $v$ associated with the absorption and emission of the photon can be calculated as follows $$
\begin{aligned}
\quad v & =\frac{\Delta E}{h}=\frac{R_{H}}{h}\left[\frac{1}{n_{i}^{2}}-\frac{1}{n_{f}^{2}}\right] \\
\Rightarrow \quad v & =\frac{2.18 \times 10^{-18} \mathrm{~J}}{6.626 \times 10^{-34} \mathrm{Js}}\left[\frac{1}{n_{i}^{2}}-\frac{1}{n_{f}^{2}}\right] \\
v & =3.29 \times 10^{15}\left[\frac{1}{n_{i}^{2}}-\frac{1}{n_{f}^{2}}\right] \mathrm{Hz} \\
\Rightarrow \bar{v} & =\frac{v}{c}=\frac{3.29 \times 10^{15}}{3 \times 10^{8} \mathrm{~ms}^{-1}}\left[\frac{1}{n_{i}^{2}}-\frac{1}{n_{f}^{2}}\right] \\
\bar{v} & =1.09677 \times 10^{7}\left[\frac{1}{n_{i}^{2}}-\frac{1}{n_{f}^{2}}\right] \mathrm{m}^{-1} \\
& \bar{v}=109677\left[\frac{1}{n_{i}^{2}}-\frac{1}{n_{f}^{2}}\right] \mathrm{cm}^{-1}
\end{aligned}
$$Show Answer
Answer In hydrogen spectrum, the spectral lines are expressed in term of wave number $\bar{v}$ obey the following formula $$
\begin{aligned}
& \text{Wave number}, \bar{v}=R_{H}\left(\frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}}\right) \quad\left(\text { where, } R_{H}=\text { Rydberg constant } 109677 \mathrm{~cm}^{-1}\right) \\
& \bar{v}=109677 \mathrm{~cm}^{-1}\left(\frac{1}{2^{2}}-\frac{1}{3^{2}}\right) \\
& \bar{v}=109677 \times \frac{5}{36}=15232.9 \mathrm{~cm}^{-1} \\
& \bar{v}=\frac{1}{\lambda}
\end{aligned}
$$ $$
\begin{aligned}
& \text { or, } \quad \lambda=\frac{1}{v}=\frac{1}{15232.9}=6.564 \times 10^{-5} \mathrm{~cm} \\
& \text { Wavelength, } \quad \lambda=6.564 \times 10^{-7} \mathrm{~m} \\
& \text { Energy, } \quad E=\frac{h c}{\lambda} \\
& =\frac{6.626 \times 10^{-34} \mathrm{Js} \times 3.0 \times 10^{8} \mathrm{~ms}^{-1}}{6.564 \times 10^{-7} \mathrm{~m}} \\
& =3.028 \times 10^{-19} \mathrm{~J} \\
& \text { Frequency, } \quad v=\frac{c}{\lambda}=\frac{3.0 \times 10^{8} \mathrm{~ms}^{-1}}{6.564 \times 10^{-7} \mathrm{~m}} \\
& =0.457 \times 10^{15} \mathrm{~s}^{-1}=4.57 \times 10^{14} \mathrm{~s}^{-1}
\end{aligned}
$$ Note When an electron returns from $n_{2}$ to $n_{1}$ state, the number of lines in the spectrum will be equal to $\frac{\left(n_{2}-n_{1}\right)\left(n_{2}-n_{1}+1\right)}{2}$.Show Answer
Show Answer
Answer
In Bohr model, an electron is regarded as a charged particle moving in well defined circular orbits about the nucleus. An orbit can completely be defined only if both the position and the velocity of the electron are known exactly at the same time.
This is not possible according to the Heisenberg uncertainty principle. Further more, the wave character of the electron is not considered in Bohr model.
Therefore, concept of movement of an electron in an orbit was replaced by the concept of probabitlity of finding electron in an orbital due to de-Broglie concept of dual nature of electron and Heisenberg’s uncertainty principle. The changed model is called quantum mechanical model of the atom.










