Properties of Matter 2 Question 23
23. A column of mercury of length
(1978)
Show Answer
Answer:
Correct Answer: 23. 2.95 cm
Solution:
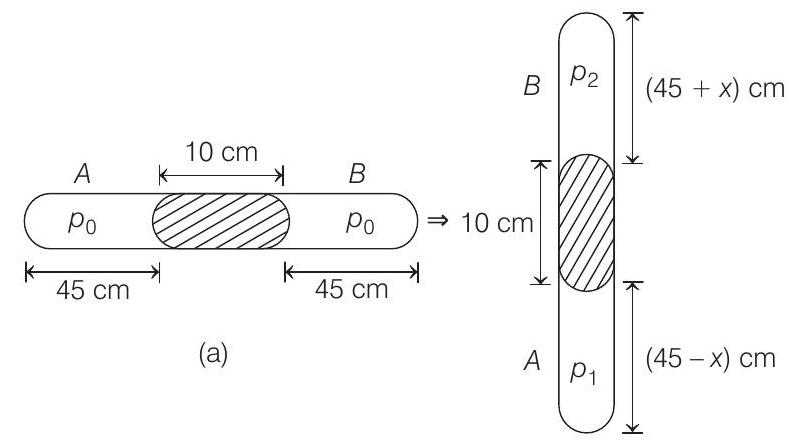
- Let area of cross-section of the tube be
(b)
Applying
From Eqs. (i) and (ii), we get
From figure (b),
or
Solving this equation, we get






