Heat and Thermodynamics 5 Question 35
36. An ideal monoatomic gas is confined in a horizontal cylinder by a spring loaded piston (as shown in the figure). Initially the gas is at temperature
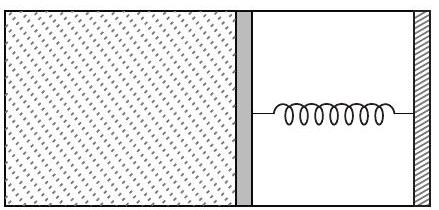
(2015 Adv.)
(a) If
then the energy stored in the spring is
(b) If
then the change in internal energy is
(c) If
then the work done by the gas is
(d) If
then the heat supplied to the gas is
Show Answer
Answer:
Correct Answer: 36.
Solution:
- Note This question can be solved if right hand side hand side chamber is assumed open, so that its pressure remains constant even if the piston shifts towards right.
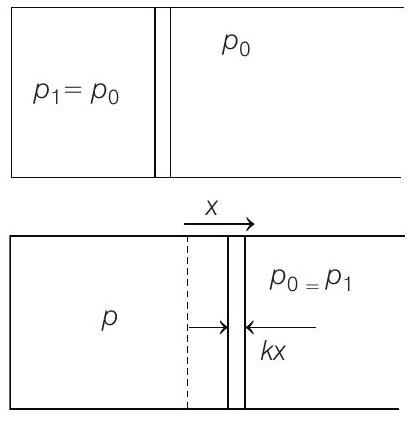
(a)
Temperature is made three times and volume is doubled
Further
Energy of spring
(b)
(c)
(d)
NOTE






