Heat and Thermodynamics 5 Question 11
11. Three Carnot engines operate in series between a heat source at a temperature $T _1$ and a heat sink at temperature $T _4$ (see figure). There are two other reservoirs at temperatures $T _2$ and $T _3$, as shown with $T _1>T _2>T _3>T _4$. The three engines are equally efficient if
(Main 2019, 10 Jan I)
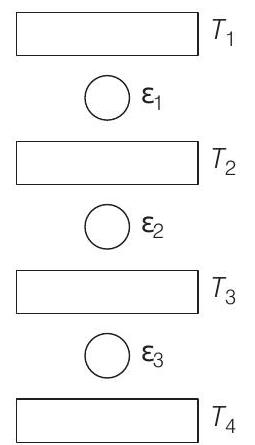
(a) $T _2=\left(T _1^{3} T _4\right)^{1 / 4} ; T _3=\left(T _1 T _4^{3}\right)^{1 / 4}$
(b) $T _2=\left(T _1^{2} T _4\right)^{1 / 3} ; T _3=\left(T _1 T _4^{2}\right)^{1 / 3}$
(c) $T _2=\left(T _1 T _4\right)^{1 / 2} ; T _3=\left(T _1^{2} T _4\right)^{1 / 3}$
(d) $T _2=\left(T _1 T _4^{2}\right)^{1 / 3} ; T _3=\left(T _1^{2} T _4\right)^{1 / 3}$
Show Answer
Answer:
Correct Answer: 11. (b)
Solution:
- Given, Carnot engines operates as,
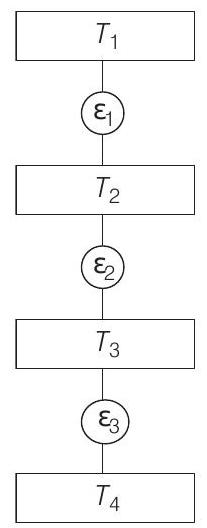
As, efficiency of a Carnot’s engine is given by
We have,
$$ \eta=1-\frac{T _{\text {sink }}}{T _{\text {source }}} $$
$\eta _1=$ efficiency of engine $\varepsilon _1=1-\frac{T _2}{T _1}$
$\eta _2=$ efficiency of engine $\varepsilon _2=1-\frac{T _3}{T _2}$ $\eta _3=$ efficiency of engine $\varepsilon _3=1-\frac{T _4}{T _3}$
For equal efficiencies,
$$ \begin{array}{rlrl} & \eta _1=\eta _2=\eta _3 \\ \Rightarrow \quad 1-\frac{T _2}{T _1} & =1-\frac{T _3}{T _2}=1-\frac{T _4}{T _3} \\ \Rightarrow \quad & \frac{T _2}{T _1} & =\frac{T _3}{T _2}=\frac{T _4}{T _3} \\ \Rightarrow \quad & T _2^{2} & =T _1 T _3 \text { and } T _3^{2}=T _2 T _4 \\ \Rightarrow \quad T _2^{4} & =T _1^{2} T _3^{2} \\ & & T _2^{4} & =T _1^{2} T _2 T _4 \\ \text { or } & T _2^{3} & =T _4 T _1^{2} \\ \text { Also, } & T _2 & =\left(T _1^{2} T _4\right)^{\frac{1}{3}} \\ & & T _3^{4} & =T _2^{2} T _4^{2}=T _1 T _3 T _4^{2} \\ & & T _3^{3} & =T _1 T _4^{2} \text { or } T _3=\left(T _1 T _4^{2}\right)^{\frac{1}{3}} \end{array} $$






