Heat and Thermodynamics 5 Question 11
11. Three Carnot engines operate in series between a heat source at a temperature
(Main 2019, 10 Jan I)
(a)
(b)
(c)
(d)
Show Answer
Solution:
- Given, Carnot engines operates as,
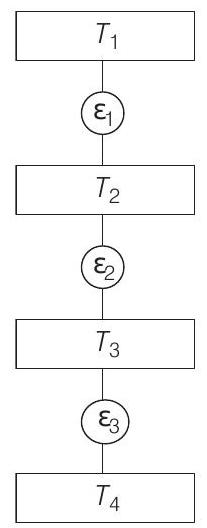
As, efficiency of a Carnot’s engine is given by
We have,
For equal efficiencies,
Key idea In a Carnot engine the heat flow from higher temperature source
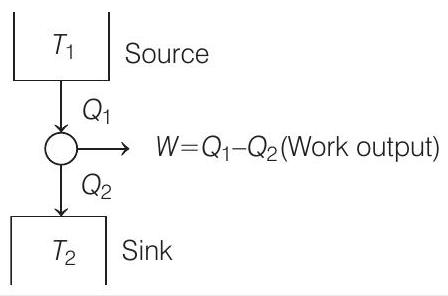
For the given condition, Carnot engine
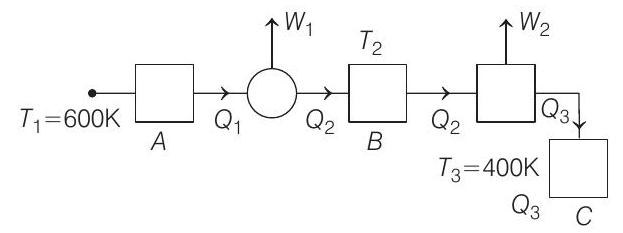
where,
According to Carnot engine principle,
As per the given condition, if the work outputs of the two engines are equal, then






