D And F Block Elements
TRANSITION ELEMENTS AND COMPLEXES
In the long form of periodic table elements are classified based on electronic configuration. The elements which are classified between’s’ and ’ $p$ ’ block are ’ $d$ ’ block elements or transition elements. In these elements the differentiating electron enters in the ’d’ orbitals of penultimate shell.
-
General configuration for ’ $d$ ’ block elements is $n s^{1-2}(n-1) d^{1-10}$. i.e., in ’ $d$ ’ block elements the valence shell has constant number of electrons whereas the number of electron in penultimate shell go on increasing.
-
Elements which have at least one unpaired electron in their ’ $\mathrm{d}$ ’ orbital in atomic or any oxidation state are called as Transition elements. Thus all transition elements are ’ $d$ ’ block elements but all ’ $d$ ’ block elements may not be transition elements or the elements having incompletely filled ’ $d$ ’ orbitals are called as transition elements.
-
Transition elements are classified between ’s’ and ’ $p$ ’ blocks from fourth period onwards. Series of transition elements are four
-
$1^{\text {st }}$ Series - They are classified in fourth period and are called as ‘3d’ series of elements. Their atomic numbers are $21(\mathrm{Sc})$ to $30(\mathrm{Zn})$.
-
$2^{\text {nd }}$ Series- They are classified in fifth period and are called as ’ $4 d^{\prime}$ ’ series of elements. Their atomic numbers are 39 (Y) to 48 (Cd).
-
$3^{\text {rd }}$ series- They are classified in sixth period and are called as ’ $5 \mathrm{d}$ ’ series of elements. Their atomic numbers are $57(\mathrm{La}), 72(\mathrm{Hf})$ to $80(\mathrm{Hg})$
-
$4^{\text {th }}$ Series - They are classified in the seventh period and are called as ’ $6 \mathrm{d}$ ’ series of elements. This is an incomplete series. Their atomic numbers are 89(Ac), 104(Ku) to 112 (Uub).
-
Electronic configuration of first series of transition elements.
| Atomic number | Element | Symbol | Electronic configuration |
|---|---|---|---|
| 21. | Scandium | Sc | [Ar] $4s^2$ $3d^1$ |
| 22. | Titanium | Ti | [Ar] $4s^2$ $3d^2$ |
| 23. | Vanadium | V | [Ar] $4s^2$ $3d^3$ |
| 24. | Chromium | Cr | [Ar] $4s^1$ $3d^5$ |
| 25. | Manganese | Mn | [Ar] $4s^2$ $3d^5$ |
| 26. | Iron | Fe | [Ar] $4s^2$ $3d^6$ |
| 27. | Cobalt | Co | [Ar] $4s^2$ $3d^7$ |
| 28. | Nickel | Ni | [Ar] $4s^2$ $3d^8$ |
| 29. | Copper | Cu | [Ar] $4s^1$ $3d^{10}$ |
| 30. | Zinc | Zn | [Ar] $4s^2$ $3d^{10}$ |
-
Chromium and copper are elements having exceptional electronic configurations of $[\mathrm{Ar}] 4 s^1 3 d^5$ and $[\mathrm{Ar}] 4 s^1 3 d^{10}$ instead of $[\mathrm{Ar}] 4 \mathrm{~s}^2 3 \mathrm{d}^4$ and $[\mathrm{Ar}] 4 \mathrm{~s}^2 3 \mathrm{d}^9$
-
$\mathrm{Zn}(30)$ is $[\mathrm{Ar}] 4 \mathrm{~s}^2 3 \mathrm{d}^{10}$
$\mathrm{Cd}(48)$ is $[\mathrm{Kr}] 5 \mathrm{~s}^2 4 \mathrm{d}^{10}$
$\mathrm{Hg}(80)$ is $[\mathrm{Xe}] 6 \mathrm{~s}^2 4 \mathrm{f}^{14} 5 \mathrm{d}^{10}$
These three elements do not have any unpaired electrons in their ’ $d$ ’ orbitals in atomic as well as in ionic states. Therefore they are only classified as ’d’ block elements and not as transition elements. Copper, Silver and Gold, the elements of IB group i.e., coinage metals, have $n s^1(n-1) d^{10}$ configuration. They are transition elements as in their higher oxidation state they have an unpaired electron in their ’ $d$ ’ orbitals.
Melting and boiling points
-
M.p and B.p of d-block > s-block (the reason is stronger metallic bond and presence of covalent bond formed by unpaired d-electrons.)
-
In $\mathrm{Zn}, \mathrm{Cd}$, and $\mathrm{Hg}$ there is no unpaired electron present in d-orbital, hence due to absence of covalent bond, their m.p and b.p are very low. (Volatile metals $\mathrm{Zn}, \mathrm{Cd}, \mathrm{Hg}$ )
-
In 3d series $\mathrm{Sc} \rightarrow \mathrm{Cr}$ m.p,b.p increases $\mathrm{Mn}>\mathrm{Zn}$ m.p, b.p decreases Mn and Tc possess comparatively low m.p . It is due to stable configuration’ (Half filled) Lowest $\mathrm{mp} \mathrm{Hg}=38^{\circ} \mathrm{C}$ Highest mp. $\mathrm{W}=3400^{\circ} \mathrm{C}$
Characteristic properties of transition elements are
(a) Variable oxidation state
(b) Coloured ions
(c) Paramagnetic properties
(d) Catalytic properties
(e) Formation of alloys
(f) Formation of interstitial compounds
(g) Formation of complexes
Variable valency or variable oxidation states:
PYQ-2023- d and f block elements-Q7
They exhibit variable valency due to involvement of $( n s)$ and ( $n-1$ )d electrons in bonding. This is due to less energy difference between these electrons.
- The oxidation states of all transition elements of ’ $3 \mathrm{d}$ ’ series are as follows
| Element | Electronic Configuration | Oxidation States |
|---|---|---|
| Sc | [Ar] $4s^2 3d^1$ | +3 |
| Ti | [Ar] $4s^2 3d^2$ | +2, +3, +4 |
| V | [Ar] $4s^2 3d^3$ | +2, +3, +4, +5 |
| Cr | [Ar] $4s^1 3d^5$ | +1, +2, +3, +4, +5, +6 |
| Mn | [Ar] $4s^2 3d^5$ | +2, +3, +4, +5, +6, +7 |
| Fe | [Ar] $4s^2 3d^6$ | +2, +3, +4, +5, +6 |
| Co | [Ar] $4s^2 3d^7$ | +2, +3, +4 |
| Ni | [Ar] $4s^2 3d^8$ | +2, +3, +4 |
| Cu | [Ar] $4s^1 3d^10$ | +1, +2 |
-
Highest oxidation state of transition elements can be calculated by $=n+2$ ( $n=$ no. of unpaired e-) (It is not applied for $\mathrm{Cr}$ and $\mathrm{Cu}$ )
-
The transition metal ions having stable configuration are stable
-
Transition metal ions having $3 \mathrm{d}^5$ configuration are stable like $\mathrm{Mn}^{+2}, \mathrm{Fe}^{+3}$ In aqueous medium $\mathrm{Cr}^{+3}$ is stable. $\mathrm{Co}^{+2}$ and $\mathrm{Ni}^{+2}$ are stable.
-
Transition metal ion with $3 \mathrm{d}^{10}$ configuration which is stable is $\mathrm{Cu}^{+1}, \ln$ aqueous medium $\mathrm{Cu}^{+2}$ is more stable than $\mathrm{Cu}^{+1}$.
-
Most common oxidation state among the transition elements is +2 .
-
Highest oxidation state shown by transition elements of ’ $4 \mathrm{d}$ ’ and ’ $5 \mathrm{d}$ ’ series is +8 . The elements showing this oxidation state are Ruthenium (44) and Osmium (76).
-
The common oxidation state shown by elements of IIIB i.e. Sc, Y, La and Ac is + 3 as their divalent compounds are highly unstable.
- In lower oxidation state transition elements form ionic compounds and in higher oxidation state their compounds are covalent.
e.g. in chromate ion $\mathrm{CrO}_4{ }^{-2}$, the bonds between $\mathrm{Cr}$ and $\mathrm{O}$ are covalent.
- Generally higher oxidation states are exhibited in the compounds which are formed with highly electronegative elements like $O$ and $F$.
- They also shows zero oxidation state in their carbonyl compounds like $\mathrm{Ni}(\mathrm{CO})_4$
- Usually transition metal ions in their lower oxidation state act as reducing agents and in higher oxidation state they are oxidizing agents.
e.g.: $\mathrm{Ti}^{+2}, \mathrm{~V}^{+2}, \mathrm{Fe}^{+2}, \mathrm{Co}^{+2}$ etc. are reducing agents
$\mathrm{Cr}^{+6}, \mathrm{Mn}^{+7}, \mathrm{Mn}^{+4} \mathrm{Mn}^{+5}, \mathrm{Mn}^{+6}$ etc. are oxidizing agents.
Colour Property:
PYQ-2024-Coordination_Compounds-Q1
- Most of the transition metal ions exhibit colour property. This is due to the presence of unpaired electrons in their ’d’ orbitals. They require less amount of energy to undergo excitation of electrons. Hence they absorb visible region of light exhibiting colour. $\mathrm{Ti}^{+2}[\mathrm{Ar}] 3 \mathrm{d}^2, \mathrm{~V}^{+2}[\mathrm{Ar}] 3 \mathrm{d}^3$ etc.
These are having unpaired electrons in their ’ $d$ ’ orbitals therefore they are coloured.
- Transition metal ions which do not have any unpaired elctrons in their ’ $d$ ’ orbitals like $3 d^0$ and $3 d^{10}$ configurations, do not exhibit any colour property.
e.g., $\mathrm{Sc}^{+3}[\mathrm{Ar}] 3 \mathrm{d}^0, \mathrm{Cu}^{+1}[\mathrm{Ar}] 3 \mathrm{d}^{10}, \mathrm{Ti}^{+4}[\mathrm{Ar}] 3 \mathrm{d}^0$ etc. are colourless ions.
- A transition metal ion absorbs a part of visible region of light and emits rest of the six colours, the combination of which is the colour of emitted light. The colour of metal ion is the colour of the emitted light.
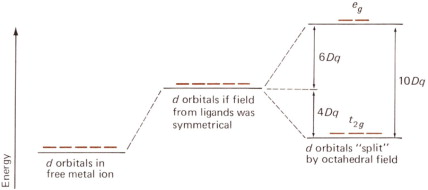
- In transition metal ion the ’d’ orbitals split into lower energy set $t_2 g$ orbitals and higher energy set $e_g$ orbitals. The electrons from $t_2 g$ set get excited to higher energy set i.e., eg set. This excitation of electrons is called as ’ $d-d$ ’ transition. As $d$-d transition requires less amount of energy they absorb visible region of light. Due to this ’ $d$ - $d$ ’ transition the transition metal ions exhibit colour property.
Lower energy set $=\mathrm{t}_2 \mathrm{g}$
Higher energy set $=$ $e_g$
- $\quad \mathrm{KMnO}_4$ (dark pink), $\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7$ (orange) having do configuration are coloured due to charge transfer spectrum.
Some of the coloured metal ions are as follows :
| Ion | Color | Ion | Color |
|---|---|---|---|
| $Ti^{3+}$ | Purple | $Cr^3+$ | Green |
| $Mn^2+$ | Light pink | $Fe^2+ $ | Pale green |
| $Fe^3+$ | Yellow | $Co^2+$ | Blue |
| $Ni^2+$ | Green | $Cu^2+$ | Blue |
Magnetic properties
- Matter, in general is associated with magnetic properties. Majority of substances are either paramagnetic or diamagnetic. A paramagnetic substance is one which is attracted into a magnetic field. Paramagnetism is mainly due to the presence of unpaired electrons in atoms or ions or molecules. Diamagnetic substance is one which is slightly repelled by a magnetic field.
$$ \mathrm{Ti}^{+2}[\mathrm{Ar}] 3 \mathrm{d}^2, \mathrm{Ti}^{+3}[\mathrm{Ar}] 3 \mathrm{d}^1 . \mathrm{V}^{+2}[\mathrm{Ar}] 3 \mathrm{d}^3, \mathrm{Cr}^{+3}[\mathrm{Ar}] 3 \mathrm{d}^3 $$
As is evident most of the transition metal ions have unpaired electrons in their ’ $d$ ’ orbitals. Hence most of the transition metal ions are paramagnetic in nature. Transition metal ions having $3 d^0$ and $3 d^{10}$ configuration exhibit diamagnetic nature.
- An unpaired electron spins and as it is a charged particle, magnetic field is created due to its spinning.
- Each electron may, in fact, be considered as a micro magnet having a certain value of magnetic moment. The total magnetic moment of a substance is the resultant of the magnetic moments of all the individual electrons. Thus substances containing unpaired electrons get attracted towards the magnets exhibiting paramagnetic nature.
The magnetic moment ( $\mu$ ) created due to spinning of unpaired electrons can be calculated by using
PYQ-2023- d and f block elements-Q1
$\mu=\sqrt{n(n+2)}: \quad$ Where ’ $n$ ’ is the number of unpaired electrons in the metal ion. $$ \mu=\text { Magnetic moment in Bohr Magnetons (B.M.) } $$
-
The magnetic moment of diamagnetic substances will be zero.
-
As the number of unpaired electrons increase the magnetic moment created goes on increasing and hence the paramagnetic nature also increases.
-
Transition metal ions having $d^5$ configuration will have maximum number of unpaired electrons therefore they will be maximum paramagnetic in nature.
Catalytic Property
-
Transition elements and their compounds exhibit catalytic properties. This is due to their variable valency as well as due to the free valencies on their surface.
-
When transition elements and their compounds are in powdered state. Their catalytic properties exhibited will be to a greater extent. This is due to greater surface area available in the powdered state.
-
Transition metals and their compounds exhibiting catalytic properties in various processes are
(i) $\mathrm{Fe}$ is used in Haber’s process for manufacture. of $\mathrm{NH}_3$
(ii) $\mathrm{V}_2 \mathrm{O}_5$ is used in contact process for $\mathrm{H}_2 \mathrm{SO}_4$ manufacture
(iii) $\mathrm{Pt}$ is used in Ostwald’s process of nitric acid
(iv) $\mathrm{Ni}$ is used in hydrogenation of oils
(v) $\mathrm{FeSO}_4$ is used in oxidation of Benzene with $\mathrm{H}_2 \mathrm{O}_2$
(vi) $\mathrm{Cu}$ is used in dehydrogenation of alcohols
(vii) $\mathrm{TiCl}_4$ is used as catalyst in Vinyl polymerization.
Formation of Alloy
- Transition elements have maximum tendency to form alloys.
- The reactivity of transition elements is very less and their sizes are almost similar. Due to this a transition metal atom in the Lattice can be easily replaced by other transition metal atom and hence they have maximum tendency to form alloys.
- In the alloys ratio of component metals is fixed.
- These are extremely hard and have high melting point.
SOME IMPORTANT ALLOY
- Bronze - ${Cu}(75-90 %)+{Sn}(10-25%)$
- Brass - ${Cu}(60-80 %)+{Zn}(20-40%)$
- Gun metal- $({Cu}+{Zn}+{Sn})(87: 3: 10)$
- German Silver- ${Cu}+{Zn}+{Ni}(2: 1: 1)$
- Bell metal - ${Cu}(80 %)+{Sn}(20 %)$
- Nichrome- $({Ni}+{Cr}+{Fe})$
- Alnico- $({Al},{Ni},{Co})$
- Type Metal- ${Pb}+{Sn}+{Sb}$
Trends in the $M^{2+}/M$ Standard Electrode Potentials
| Dlement (M) | $\boldsymbol{\Delta}_{\mathbf{a}} \boldsymbol{H}^{\ominus}(\mathbf{M})$ | $\boldsymbol{\Delta}_{\mathbf{i}} \mathbf{H}_1^{\ominus}$ | $\boldsymbol{\Delta}_{\mathbf{1}} \mathbf{H}_2^{\ominus}$ | $\boldsymbol{\Delta}_{\mathrm{hyd}} \mathbf{H}^{\ominus}\left(\mathbf{M}^{2+}\right)$ | $\mathbf{E}^{\ominus} / \mathbf{V}$ |
|---|---|---|---|---|---|
| $\mathrm{Ti}$ | 469 | 656 | 1309 | -1866 | -1.63 |
| $\mathrm{~V}$ | 515 | 650 | 1414 | -1895 | -1.18 |
| $\mathrm{Cr}$ | 398 | 653 | 1592 | -1925 | -0.90 |
| $\mathrm{Mn}$ | 279 | 717 | 1509 | -1862 | -1.18 |
| $\mathrm{Fe}$ | 418 | 762 | 1561 | -1998 | -0.44 |
| $\mathrm{Co}$ | 427 | 758 | 1644 | -2079 | -0.28 |
| $\mathrm{Ni}$ | 431 | 736 | 1752 | -2121 | -0.25 |
| $\mathrm{Cu}$ | 339 | 745 | 1958 | -2121 | 0.34 |
| $\mathrm{Zn}$ | 130 | 906 | 1734 | -2059 | -0.76 |
- The unique behaviour of $\mathrm{Cu}$, having a positive $E^{\ominus}$, accounts for its inability to liberate $\mathrm{H}_2$ from acids.
- Only oxidising acids (nitric and hot concentrated sulphuric) react with $\mathrm{Cu}$, the acids being reduced.
- The high energy to transform $\mathrm{Cu}(\mathrm{s})$ to $\mathrm{Cu}^{2+}(\mathrm{aq})$ is not balanced by its hydration enthalpy. The general trend towards less negative $E^{\ominus}$ values across the electrode potentials

Trends in the $M^{3+}/M^{2+}$ Standard Electrode Potentials
$E^{\ominus}\left(\mathrm{M}^{3+} / \mathrm{M}^{2+}\right)$ values shows the varying trends
- The low value for $\mathrm{Sc}$ reflects the stability of $\mathrm{Sc}^{3+}$ which has a noble gas configuration.
- The highest value for $\mathrm{Zn}$ is due to the removal of an electron from the stable $d^{10}$ configuration of $\mathrm{Zn}^{2+}$.
- The comparatively high value for $\mathrm{Mn}$ shows that $\operatorname{Mn}^{2+}\left(d^5\right)$ is particularly stable, whereas comparatively low value for $\mathrm{Fe}$ shows the extra stability of $\mathrm{Fe}^{3+}\left(d^5\right)$.
- The comparatively low value for $\mathrm{V}$ is related to the stability of $\mathrm{V}^{2+}$ (half-filled $t_{2 \mathrm{~g}}$ level.
Trends in Stability of Higher Oxidation States
-
The highest oxidation numbers are achieved in $\mathrm{TiX}_4$ (tetrahalides), $\mathrm{VF}_5$ and $\mathrm{CrF}_6$.
-
The +7 state for $\mathrm{Mn}$ is not represented in simple halides but $\mathrm{MnO}_3 \mathrm{~F}$ is known, and beyond $\mathrm{Mn}$ no metal has a trihalide except $\mathrm{FeX}_3$ and $\mathrm{CoF}_3$.
-
The ability of fluorine to stabilise the highest oxidation state is due to either higher lattice energy as in the case of $\mathrm{CoF}_3$, or higher bond enthalpy terms for the higher covalent compounds, e.g., $\mathrm{VF}_5$ and $\mathrm{CrF}_6$.
-
$\mathrm{V}^{+5}$ is represented only by $\mathrm{VF}_5$, the other halides, undergo hydrolysis to give oxohalides, $\mathrm{VOX}_3$.
-
Another feature of fluorides is their instability in the low oxidation states e.g., $\mathrm{VX}_2(\mathrm{X}=\mathrm{CI}, \mathrm{Br}$ or $\mathrm{I})$ and the same applies to $\mathrm{CuX}$.
-
All $\mathrm{Cu}^{\mathrm{II}}$ halides are known except the iodide. In this case, $\mathrm{Cu}^{2+}$ oxidises $\mathrm{I}^{-}$to $\mathrm{I}_2$ : $$ 2 \mathrm{Cu}^{2+}+4 \mathrm{I}^{-} \rightarrow \mathrm{Cu}_2 \mathrm{I}_2(\mathrm{~s})+\mathrm{I}_2 $$
-
Many copper (I) compounds are unstable in aqueous solution and undergo disproportionation. $$ 2 \mathrm{Cu}^{+} \rightarrow \mathrm{Cu}^{2+}+\mathrm{Cu} $$
-
The stability of $\mathrm{Cu}^{2+}$ (aq) rather than $\mathrm{Cu}^{+}(\mathrm{aq})$ is due to the much more negative $\Delta_{\text {hyd }} \mathrm{H}^{\ominus}$ of $\mathrm{Cu}^{2+}$ (aq) than $\mathrm{Cu}^{+}$, which more than compensates for the second ionisation enthalpy of $\mathrm{Cu}$.
PYQ-2023- d and f block elements-Q15
The highest oxidation number in the oxides coincides with the group number and is attained in $\mathrm{Sc}_2 \mathrm{O}_3$ to $\mathrm{Mn}_2 \mathrm{O}_7$.
- Beyond Group 7, no higher oxides of $\mathrm{Fe}$ above $\mathrm{Fe}_2 \mathrm{O}_3$, are known, although ferrates $(\mathrm{VI})\left(\mathrm{FeO}_4\right)^{2-}$, are formed in alkaline media but they readily decompose to $\mathrm{Fe}_2 \mathrm{O}_3$ and $\mathrm{O}_2$.
- Oxocations stabilise $\mathrm{V}^{\mathrm{V}}$ as $\mathrm{VO}_2^{+}, \mathrm{V}^{\mathrm{IV}}$ as $\mathrm{VO}^{2+}$ and $\mathrm{Ti}^{2 \mathrm{~V}}$ as $\mathrm{TiO}^{2+}$.
PYQ-2023- d and f block elements-Q11
- The ability of oxygen to stabilise these high oxidation states exceeds that of fluorine. Thus the highest $\mathrm{Mn}$ fluoride is $\mathrm{MnF}_4$ whereas the highest oxide is $\mathrm{Mn}_2 \mathrm{O}_7$. The ability of oxygen to form multiple bonds to metals explains its superiority.
- In the covalent oxide $\mathrm{Mn}_2 \mathrm{O}_7$, each $\mathrm{Mn}$ is tetrahedrally surrounded by O’s including a Mn-O-Mn bridge. The tetrahedral $\left[\mathrm{MO}_4\right]^{\mathrm{n}-}$ ions are known for $\mathrm{V}^{\mathrm{V}}, \mathrm{Cr}^{\mathrm{V}}, \mathrm{Mn}^{\mathrm{v}}, \mathrm{Mn}^{\mathrm{V} 1}$ and $\mathrm{Mn}^{\mathrm{VII}}$.
PYQ-2023- d and f block elements-Q12
f - Block Elements
They were earlier called as rare earth metals as it was believed that they exist in earth’s crust to a very less extent for e.g.: Pm, does not exist in the earth’s crust. But this terminology is now not applicable as they exist in earth’s crust to a sufficient extent.
INNER TRANSITION ELEMENTS
The elements in which the additional electron enters in $(n-2)$ f orbitals are called inner transition elements. or f-block elements.
Position in the periodic table
The lanthanides resemble yttrium in most of their properties. So it became necessary to accommodate all the fifteen elements together at one place. This has been done by placing the first element, lanthanum below yttrium and placing the remaining fourteen elements separately in the lower part of the periodic table. Lanthanide series $\quad (Z= 58-71) \quad (Ce-Lu)$
Actinide series $\quad (Z=90-103) \quad (Th-Lw)$
Lanthanides
Lanthanides are reactive elements so do not found in free state in nature. Most important minerals for lighter Lanthanides are - Monazite, cerite and orthite and for heavier lanthanides - Gadolinite and Xenotime Electronic configuration
The general configuration of lanthanides may be given as $4 f^{2-14} 5 s^2 5 p^6 5 d^{0 / 1} 6 s^2$. Lanthanoid have outer three shells incomplete.
| Atomic Number | Element | Symbol | Outer Electronic Atomic Configuration | +3 ion |
|---|---|---|---|---|
| 58 | Cerium | Ce | $4f^2 6s^2$ | $4f^1$ |
| 59 | Praseodymium | Pr | $4f^3 6s^2$ | $4f^2$ |
| 60. | Neodymium | Nd | $4f^4 6s^2$ | $4f^3$ |
| 61. | Promethium | Pm | $4f^5 6s^2$ | $4f^4$ |
| 62. | Samarium | Sm | $4f^6 6s^2$ | $4f^5$ |
| 63. | Europium | Eu | $4f^7 6s^2$ | $4f^6$ |
| 64. | Gadolinium | Gd | $4f^7 5d^1 6s^2$ | $4f^7$ |
| 65. | Terbium | Tb | $4f^9 6s^2$ | $4f^6$ |
| 66. | Dysprosium | Dy | $4f^{10} 6s^2$ | $4f^9$ |
| 67. | Holmium | Ho | $4f^{11} 6s^2$ | $4f^{10}$ |
| 68. | Erbium | Er | $4f^{12} 6s^2$ | $4f^{11}$ |
| 69. | Thulium | Tm | $4f^{13} 6s^2$ | $4f^{12}$ |
| 70. | Ytterbium | Yb | $4f^{14} 6s^2$ | $4f^{13}$ |
| 71. | Lutecium | Lu | $4f^{14} 5d^1 6s^2$ | $4f^{14}$ |
It is to be noted here that filling of $4 \mathrm{f}$ orbitals in the atoms is not regular. A 5d electron appears in gadolinium ( $\mathrm{z}$ $=64$ ) with an outer electronic configuration of $4 f^7 5 d^1 6 s^2$ (and not $4 f^6 6 s^2$ ). This is because the $4 f$ and $5 d$ electrons are at about the same potential energy and that the atoms have a tendency to retain stable half filled configuration.
On the other hand, the filling of $f$ orbitals is regular in tri-positive ions.
After losing outer electrons, the f orbitals shrink in size and became more stable. Pm is the only synthetic radioactive lanthanide.
Oxidation states
PYQ-2023- d and f block elements-Q3
| Lanthanides | Oxidation States |
|---|---|
| Ce (58) | +3, +4 |
| Pr (59) | +3, (+4) |
| Nd (60) | +3 |
| Pm (61) | +3 |
| Sm (62) | (+2), +3 |
| Eu (63) | +2, +3 |
| Gd (64) | +3 |
| Tb (65) | +3, +4 |
| Dy (66) | +3 ; (+4) |
| Ho (67) | +3 |
| Er (68) | (+2), +3 |
| Tm (69) | (+2), +3 |
| Yb (70) | +2, +3 |
| Lu (71) | +3 |
- Oxidation states in brackets are unstable states
- The lanthanides contains two s electrons in the outermost shell, they are therefore expected to exhibit a characteristic oxidation state of +2 . But for the lanthanides, the +3 oxidation is common.
- This corresponds to the use of two outermost electrons $\left(6 s^2\right)$ along with one inner electron. The inner electron used is a 5d electron (in La, Gd and Lu), or one of the 4 f electron if no 5 d electrons present.
- All the lanthanides attains +3 oxidation state and only cerium, praseodymium, and terbium exhibit higher oxidation state (+4).
Oxidation states +2 and +4 occur particularly when they lead to
(i) A noble gas configuration e.g. $\mathrm{Ce}^{4+}\left(f^0\right)$
(ii) A half filled ’ $f$ ’ orbital e.g. $\mathrm{Eu}^{2+}, \mathrm{Tb}^{4+},\left(f^7\right)$
(iii) A completely filled ’ $f$ ’ orbital e.g. $\mathrm{Yb}^{2+}$ (f $\left.{ }^{14}\right)$
Therefore, in higher oxidation state, they act as oxidizing while in lower state as reducing agents.
Magnetic properties
In tri-positive lanthanide ions the number of unpaired electrons regularly increases from lanthanum to Gadolinium ( 0 to 7 ) and then continuously decreases upto lutecium ( 7 to 0 ). So lanthanum and lutecium ions which are diamagnetic, all other tri-positive lanthanide ions are Paramagnetic.
Colour - The lanthanide ions have unpaired electrons in their $4 \mathrm{f}$ orbitals. Thus these ions absorbs visible region of light and undergo $f-f$ transition and hence exhibit colour. The colour exhibited depends on the number of unpaired electrons in the $4 f$ orbitals. The ions often with $4 f^n$ configuration have similar colour to those ions having $4 f^{14-n}$ configuration.
Other Properties
(a) Highly dense metals with high melting points (do not show any regular trend).
(b) Ionization Energies - Lanthanides have fairly low ionization energies comparable to alkaline earth metals.
(c) Electro positive Character - High due to low I.P.
(d) Complex formation - Do not have much tendency to form complexes due to low charge density because of their large size.
${Lu}^{+3}$ is smallest in size can only form complex.
(e) Reducing Agent - They readily lose electrons so are good reducing agent.
- In +3 oxidation states, nitrates, perchlorates and sulphates of lanthanides and actinides are water soluble, while their hydroxides, fluorides and carbonates are water insoluble.
- Alloys of lanthanides with Fe are called misch metals.
- $\mathrm{La}(\mathrm{OH})_3$ is most basic in nature while $\mathrm{Lu}(\mathrm{OH})_3$ least basic.
- Lanthanides form $\mathrm{MC}_2$ type carbide with carbon, which on hydrolysis gives $\mathrm{C}_2 \mathrm{H}_2$.
LANTHANIDE CONTRACTION
In the lanthanide series with increasing atomic number, there is a progressive decrease in the size from lanthanum to lutecium or from $\mathrm{La}^{+3}$ to $\mathrm{Lu}^{+3}$. This contraction in size is known as lanthanide contraction.
- The general electronic configuration of these elements is $4 f^{0-14} 5 s^2 p^6 d^{0-1} 6 s^2$. In these elements the added electron enters the deep seated f-orbitals and therefore experiences considerable pull by the nucleus.
- Such an electron cannot add to the size of the element and also because the intervening $5 s^2 p^6 d^1$ electronic shells, it is very little screening effect on the outermost $6 s^2$ electrons. Hence with increasing atomic number, the enhanced nuclear charge leads to contraction in the size of atoms and ions.
- The atomic volumes of europium and ytterbium are unexpectedly large. The large atomic size of Eu and $\mathrm{Yb}$ suggest weaker bonding in the solid elements. Both these elements have only two electrons extra than the stable configurations (half filled, $f^7$, and completely filled, $f^{14}$ ), hence they utilize two electrons in metallic bonding as in the case with barium.
EFFECTS OF LANTHANIDE CONTRACTION
(i) Close resemblance of Lanthanides :- The general decrease in the sizes of the lanthanides with an increase in their nuclear charges result in a small increase in their ionization energies. Hence their basic and ionic nature gradually decreases from La to Lu. This also explains the variations in properties such as increased tendency for hydrolysis and formation of complex salts and decreased thermal stability. solubility of their salts.
(ii) Similarity of yttrium with lanthanides :- The properties of yttrium are so similar to the lanthanides that it is considered more a member of the lanthanide series than a congener of scandium.
(iii) Anomalous behaviour of post-lanthanides :- The following anomalies may be observed in the behaviour of post-lanthanide elements.
(a) Atomic size - The ionic radii of $\mathrm{Zr}^{+4}$ is about $9 %$ more than $\mathrm{Ti}^{+4}$. Similar trend is not maintained on passing from the second to third transition series. The ionic radius of $\mathrm{Hf}^{+4}$, instead of increasing (because of inclusion of one more electronic shell). decreases (or is virtually equal to $\mathrm{Zr}^{+4}$ ) as a consequence of the lanthanide contraction.
This explains the close similarities between the members of the second and third transition series than between the elements of the first and second series.
(b) Ionization potential and electronegativity :- The effect of lanthanide contraction is also seen in the increase in the ionization potential values and electronegativities of the elements of the third transition series, contrary to the general trend. Because of the lanthanide contraction, the post-lanthanide elements have stronger positive field and thus the electrons are held more tightly. The greater effective nuclear charge of the former make them more electronegative than the latter.
(c) High density :- Because of lanthanide contraction the atomic sizes of the post lanthanide elements become very small. consequently, the packing of atoms in their metallic crystals become so much compact that their densities are very high. The densities of the third transition series elements are almost double to those of the second series elements.
APPLICATION OF LANTHANIDES
Cerium is most useful element in the lanthanides
(a) Ceramic application - $\mathrm{CeO}_2, \mathrm{La}_2 \mathrm{O}_3, \mathrm{Nd}_2 \mathrm{O}_3$ and $\mathrm{Pr}_2 \mathrm{P}_3$ are used as decolourizing agents for glasses.
(b) $\mathrm{CeS}\left(\mathrm{m}. \mathrm{p}-2000^{\circ} \mathrm{C}\right)$ is used in the manufacture of a special type of crucibles and refractories.
(c) Lanthanide compounds like cerium molybdate, cerium tungstate are used as paints and dyes.
(d) In textile and leather industries (Ce salts).
ACTINIDES (5f - BLOCK ELEMENTS)
The elements in which the extra electron enters 5f orbitals of ( $n-2$ )th main shell are known as actinides.
- The man made eleven elements $\mathrm{Np} _{93}$ - $\mathrm{Lr} _{103}$ are placed beyond uranium in the periodic table and are collectively called trans-uranic elements.
- Th, $\mathrm{Pa}$ and $\mathrm{U}$ first three actinides are natural elements.






