Alkyl Halide and Aryl Halide
Points to remember in Alkyl halide
Nucleophilic substitution Reaction $(S_N1,S_N2)$
PYQ-2024-Haloalkanes_and_Haloarenes-Q8, PYQ-2024-Haloalkanes_and_Haloarenes-Q6, PYQ-2024-Haloalkanes_and_Haloarenes-Q4, PYQ-2023-Q1
$\mathrm{S}_{\mathrm{N}} 1$ reaction:
PYQ-2024-Haloalkanes_and_Haloarenes-Q5, PYQ-2023 Haloalkanes and Haloarenes Q1, PYQ-2023 Haloalkanes and Haloarenes Q4,
$R-X+H_2O \xrightarrow{AgNO_3} R^{+}+AgX \downarrow \longrightarrow ROH \quad$ (R may rearrange)
Alkyl halide are hydrolyzed to alcohol very slowly by water, but rapidly by silver oxide suspended in boiling water.
$S_{N}2$ reaction:
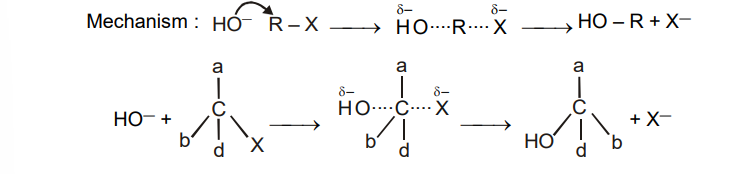
Rate of Substitution nucleophilic Reaaction
PYQ-2024-Haloalkanes_and_Haloarenes-Q10
Substitution nucleophilic bimolecular ($S_N 2$)
Of the simple alkyl halides, methyl halides react most rapidly in ($S_N 2$) reactions because there are only three small hydrogen atoms. Tertiary halides are the least reactive because bulky groups hinder the approaching nucleophiles. Thus the order of reactivity followed is: Primary halide > Secondary halide > Tertiary halide.
Substitution nucleophilic unimolecular ($S_N 1$)
Since the rate of reaction depends upon the slowest step, the rate of reaction depends only on the concentration of alkyl halide and not on the concentration of hydroxide ion. Further, greater the stability of carbocation, greater will be its ease of formation from alkyl halide and faster will be the rate of reaction. In case of alkyl halides, $3^0$ alkyl halides undergo ($S_N 1$) reaction very fast because of the high stability of $3^0$ carbocations.
For the same reasons, allylic and benzylic halides show high reactivity towards the ($S_N 1$) reaction. The carbocation thus formed gets stabilised through resonance
Elimination Reaction
PYQ-2024-Haloalkanes_and_Haloarenes-Q12, PYQ-2023 Haloalkanes and Haloarenes Q6
$CH_3-CH_2Cl \xrightarrow[Ethanol,heat] {NaOH}CH_2=CH_2 $
Halogenation Reaction
PYQ-2024-Hydrocarbons-Q21, PYQ-2024-Hydrocarbons-Q11, PYQ-2024-Haloalkanes_and_Haloarenes-Q11, PYQ-2024-Haloalkanes_and_Haloarenes-Q7, PYQ-2024-Haloalkanes_and_Haloarenes-Q2, PYQ-2023 Haloalkanes and Haloarenes Q3
$ CH_3–CH_3 + Cl_2 \xrightarrow{HCl} 2CH_3Cl$
Reaction With KCN and AgCN
PYQ-2024-Haloalkanes_and_Haloarenes-Q1
KCN is predominantly ionic and provides cyanide ions in solution. The attack takes place mainly through carbon atom and not through nitrogen atom as C-C bond is more stable than C-N bond. In contrast, AgCN is mainly covalent in nature and nitrogen is free to donate electron pair forming isocyanide as the major product.
$R-X + KCN \rightarrow R-C\equiv N + KX$
$\quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \text{Alkyl cyanide} $
$R-X + AgCN \rightarrow R-N\equiv C + AgX$
$\quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \text{Alkyl iso cyanide} $
Carbo cation stability
PYQ-2023 Haloalkanes and Haloarenes Q2
The stability of carbocations is dependent upon a few factors. One factor that decides the stability of a carbocation is resonance. Resonance is a stabilizing feature to a carbocation because it delocalizes the positive charge and creates additional bonding between adjacent atoms. Decreasing the electron deficiency increases the stability
The general rules for carbocation stability are-
(i) Increasing substitution increases stability.
$CH_3^{+}$ (methyl ; least stable) < $RCH_2^{+}(1°)$ <$R_2 CH^{+} (2°)$ < $R_3C^{+}$ (3° ; most stable)
(ii) Resonance is more important than substitution
A primary vinylic carbocation is less stable then a primary alkyl carbocation
Melting Point of DiChloroBenzene
PYQ-2023 Haloalkanes and Haloarenes Q5
- Para structure is more symmetrical, and so, it fits more effectively into the crystal lattice. Hence, it is very difficult to break it out of the lattice structure. Hence, its boiling point is the highest.
- Ortho structure involves in $+\mathbf{R}$ effect. This effect strengthens the ortho structure and hence, its boiling point is higher than meta, which does not allow $+R$ effect.






