nuclei Question 37
Question: Q. 12. (i) The number of nuclei of a given radioactive nucleus, at times
(ii) Identify the nature of the ‘radioactive radiations’, emitted in each step of the ‘decay chain’ given below :
U] [SQP 2013]
Show Answer
Solution:
Ans. (i) According to the (exponential) law of radioactive decay,
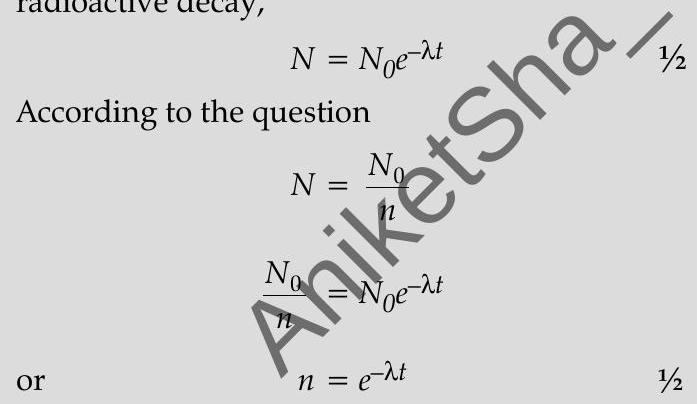
Taking
(ii) In
In
[CBSE Marking Scheme 2013]






