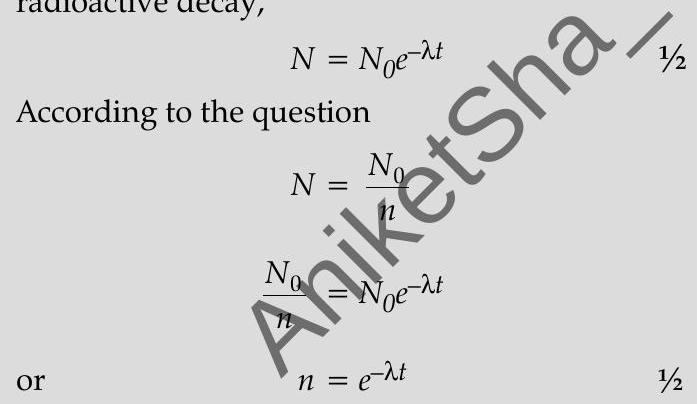Question: Q. 12. (i) The number of nuclei of a given radioactive nucleus, at times and , are and respectively. Obtain an expression for the half life of this nucleus in terms of and .
(ii) Identify the nature of the ‘radioactive radiations’, emitted in each step of the ‘decay chain’ given below :
U] [SQP 2013]
Show Answer
Solution:
Ans. (i) According to the (exponential) law of radioactive decay,
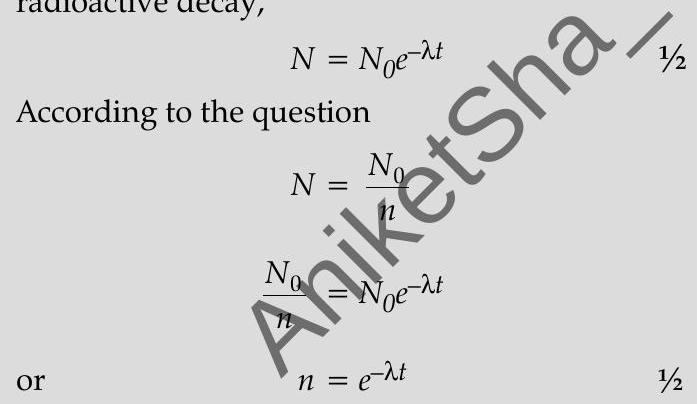
Taking on both sides
(ii) In ; Mass number changes by 4 units and atomic number changes by 2 units. Hence, it is -decay.
In ${ }^{A-4} \mathrm{Y}{\mathrm{Z}-2} \rightarrow{ }^{A-4} Y{\mathrm{Z}-2}\gamma$ - decay.
In ; there is no change in mass number and +1 change in atomic number. Hence this is decay.
[CBSE Marking Scheme 2013]