nuclei Question 35
Question: Q. 8. Half life of
Calculate the activity of 1 gram sample of
Given Avagadro’s number
Show Answer
Solution:
Ans. Activity,
Activity,
Number of nuclei present in 1 gram sample of
[CBSE Marking Scheme 2016]
Detailed Answer :
According to the question
Activity,
where,
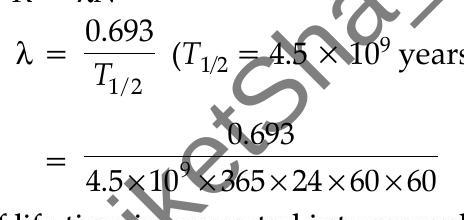
(half life time is converted into seconds)
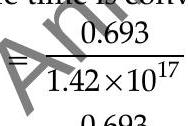
Hence,






