Chemistry Class 12 Unit 13 Chapter 02 Nitrogen Containing Organic Compounds L 2 5 7N2Ojhqr Me En Punc Para Txt
So, in continuation with our discussion on nitrogen containing organic compounds, I stopped at the stage of aromatic nitro compounds. Aromatic nitro compounds mean the benzene ring or a higher homologues of benzene like naphthalene
or a higher homologues of benzene like naphthalene , anthracene
, anthracene , phenanthrene
, phenanthrene , etc. will be there in one part, and nitrogen should be attached either inside the ring or as a substitution. So, I started with a substituted benzene, derivative or nitro benzene
, etc. will be there in one part, and nitrogen should be attached either inside the ring or as a substitution. So, I started with a substituted benzene, derivative or nitro benzene , and I said that our main aim in the first part was the preparation and use of aromatic amines. So how from aromatic nitro compound, we can prepare the aromatic amines, that is by simple reduction and that could be used by simple reagents like zinc, commercial zinc and dilute hydrochloric acid (HCl) to prepare nacent hydrogen, which in C2, can convert the nitro benzene
, and I said that our main aim in the first part was the preparation and use of aromatic amines. So how from aromatic nitro compound, we can prepare the aromatic amines, that is by simple reduction and that could be used by simple reagents like zinc, commercial zinc and dilute hydrochloric acid (HCl) to prepare nacent hydrogen, which in C2, can convert the nitro benzene to the aniline
to the aniline . So aromatic amine preparation will not be difficult if I take two step that is electrophilic substitution reaction to make the carbon-nitrogen bond and then reduction now electrophilic substitution reaction is very interesting phenomena in aromatic chemistry. The reason I said that benzene ring
. So aromatic amine preparation will not be difficult if I take two step that is electrophilic substitution reaction to make the carbon-nitrogen bond and then reduction now electrophilic substitution reaction is very interesting phenomena in aromatic chemistry. The reason I said that benzene ring is electron cloud or electron dense thing. It will form pi complex, very easily with electrophile, then it will form the sigma complex and then give the product or re-aromatize to get the product that product is nitrobenzene
is electron cloud or electron dense thing. It will form pi complex, very easily with electrophile, then it will form the sigma complex and then give the product or re-aromatize to get the product that product is nitrobenzene . Now, if I write the structure of nitro benzene
. Now, if I write the structure of nitro benzene N double bond, O and O, so what I see is that NO2 group a coordinate bond and a double bond being shown. This NO2 group will pull electrons from the benzene ring
N double bond, O and O, so what I see is that NO2 group a coordinate bond and a double bond being shown. This NO2 group will pull electrons from the benzene ring , not push so because of this pulling electron from the benzene ring
, not push so because of this pulling electron from the benzene ring . What will happen to the benzene ring
. What will happen to the benzene ring ? Benzene ring
? Benzene ring will lose the electron density and if it loses the electron density, then its carbocation character will be developed in the benzene ring
will lose the electron density and if it loses the electron density, then its carbocation character will be developed in the benzene ring , and you know if there is more nitro group in the benzene ring
, and you know if there is more nitro group in the benzene ring one simple example: I am writing here. A known compound is 2,4,6-trinitro toluene
one simple example: I am writing here. A known compound is 2,4,6-trinitro toluene . This is toluene one, two position there is a nitro group, four position there is a nitro group and six position there is a nitro group, so 2,4,6-tri nitro toluene
. This is toluene one, two position there is a nitro group, four position there is a nitro group and six position there is a nitro group, so 2,4,6-tri nitro toluene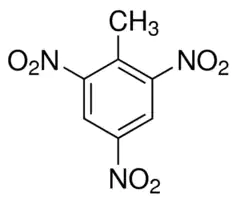 is a very important compound. You know this is for other purposes being used. This is called TNT
is a very important compound. You know this is for other purposes being used. This is called TNT  for dynamite or other compounds. These are the compounds being used and these nitro highly substituted nitro aromatics are explosive in nature. So, this is another interesting feature of carbon containing nitrogen compounds or nitrogen containing organic compounds where the nitro substituted aromatic ring. The electron density of benzene ring
for dynamite or other compounds. These are the compounds being used and these nitro highly substituted nitro aromatics are explosive in nature. So, this is another interesting feature of carbon containing nitrogen compounds or nitrogen containing organic compounds where the nitro substituted aromatic ring. The electron density of benzene ring gets reduced as a result, it becomes a carbocation in character, and you can see one very interesting feature if there be more nitro substitution, the as the electron density is decreasing. The explosive nature is also growing. One such example is TNT
gets reduced as a result, it becomes a carbocation in character, and you can see one very interesting feature if there be more nitro substitution, the as the electron density is decreasing. The explosive nature is also growing. One such example is TNT many other derivatives are there, and you know another compound. That is also very interesting in the nitro substituted thing that is phenol when being nitrated with sufficient amount of nitro group that ends up with 2,4,6-tri nitro phenol
many other derivatives are there, and you know another compound. That is also very interesting in the nitro substituted thing that is phenol when being nitrated with sufficient amount of nitro group that ends up with 2,4,6-tri nitro phenol , similar to TNT
, similar to TNT . This is called picric acid
. This is called picric acid . Picric acid
. Picric acid is also a very interesting compound, which when reacted with an aromatic compound form, a charge, transfer complex and this charge transfer complete where say, phenanthrene
is also a very interesting compound, which when reacted with an aromatic compound form, a charge, transfer complex and this charge transfer complete where say, phenanthrene or naphthalene
or naphthalene is the donor because it does not have any substitution and picric acid
is the donor because it does not have any substitution and picric acid is the acceptor to form a very nice color charge transfer complex, so those things are also used for the detection of polyaromatic hydrocarbon PAH, polyaromatic hydrocarbon. So, what does more electron, rich benzene ring
is the acceptor to form a very nice color charge transfer complex, so those things are also used for the detection of polyaromatic hydrocarbon PAH, polyaromatic hydrocarbon. So, what does more electron, rich benzene ring I have taken some other derivatives, some unusual type of structure, pyrene
I have taken some other derivatives, some unusual type of structure, pyrene , etc. so that donates the electron and nitro group containing benzene accepts the electron. That means we are able to make a benzene ring
, etc. so that donates the electron and nitro group containing benzene accepts the electron. That means we are able to make a benzene ring electron deficient by putting the electron withdrawing group on the benzene ring
electron deficient by putting the electron withdrawing group on the benzene ring . One such example is nitro, other may be fluoro or trifluoromethyl. Those groups are electron withdrawing as a result what happens? The benzene ring
. One such example is nitro, other may be fluoro or trifluoromethyl. Those groups are electron withdrawing as a result what happens? The benzene ring gets carbocation character and then it could be reacted with a nucleophile to make directly the nucleophile attacks. The benzene ring
gets carbocation character and then it could be reacted with a nucleophile to make directly the nucleophile attacks. The benzene ring and direct carbon-nitrogen bond formation will be possible. So that is a way out. Question comes how you can make the benzene ring
and direct carbon-nitrogen bond formation will be possible. So that is a way out. Question comes how you can make the benzene ring electro positive? Answer is taking up electrons with the help of electron withdrawing group like nitro, fluoro, trifluoro, and that will help and more number of electrons withdrawing group more the carbocation character will be there on the benzene ring
electro positive? Answer is taking up electrons with the help of electron withdrawing group like nitro, fluoro, trifluoro, and that will help and more number of electrons withdrawing group more the carbocation character will be there on the benzene ring and then the nucleophile should attack the way it was attacking with the aliphatic system, and so in that way the nucleophilic substitution in the benzene ring
and then the nucleophile should attack the way it was attacking with the aliphatic system, and so in that way the nucleophilic substitution in the benzene ring is also possible. That is another way to make carbon-nitrogen bond in organic chemistry. Ok now, as I want to tell you when in benzene ring
is also possible. That is another way to make carbon-nitrogen bond in organic chemistry. Ok now, as I want to tell you when in benzene ring , there is a group being present. I have taken an example of methyl group and I want to do, or I want to introduce a nitro group, because we are talking about the nitro group NO2+ introduction to the benzene ring
, there is a group being present. I have taken an example of methyl group and I want to do, or I want to introduce a nitro group, because we are talking about the nitro group NO2+ introduction to the benzene ring how we did in the previous case? We started with benzene treated with NO2+ question comes: where is the source of NO2+? You know one very interesting reaction to get the NO2+ in the textbook it is written as MA. MA is the abbreviation of mixed acid, mostly nitric acid (HNO3) and sulfuric acid (H2SO4). What concentrated nitric acid (HNO3) and sulphuric acid (H2SO4) are doing? It is generating NO2+ because sulphuric acid (H2SO4) is also a good dehydrating agent from nitric acid (HNO3). It takes off the water and generates a NO2+ or the electrophile, so electrophile is ready. The NO2+ and the benzene ring
how we did in the previous case? We started with benzene treated with NO2+ question comes: where is the source of NO2+? You know one very interesting reaction to get the NO2+ in the textbook it is written as MA. MA is the abbreviation of mixed acid, mostly nitric acid (HNO3) and sulfuric acid (H2SO4). What concentrated nitric acid (HNO3) and sulphuric acid (H2SO4) are doing? It is generating NO2+ because sulphuric acid (H2SO4) is also a good dehydrating agent from nitric acid (HNO3). It takes off the water and generates a NO2+ or the electrophile, so electrophile is ready. The NO2+ and the benzene ring is ready and they can form pi complex, sigma complex and then the substrate. So, in that way, benzene
is ready and they can form pi complex, sigma complex and then the substrate. So, in that way, benzene getting converted to nitrobenzene
getting converted to nitrobenzene very easily by an electrophilic substitution reaction, what is being substituted? The hydrogen atom of the benzene ring
very easily by an electrophilic substitution reaction, what is being substituted? The hydrogen atom of the benzene ring one of the hydrogen atoms of the benzene ring
one of the hydrogen atoms of the benzene ring , who is entering NO2+, and by that way we are able to make nitrobenzene
, who is entering NO2+, and by that way we are able to make nitrobenzene . My question is: if a methyl group is present there and now you are doing a MA or mixed acid treatment that is NO2+ being generated and being introduced where it will go that terminology to know where the second group will go when there Is a mono substituted benzene ring
. My question is: if a methyl group is present there and now you are doing a MA or mixed acid treatment that is NO2+ being generated and being introduced where it will go that terminology to know where the second group will go when there Is a mono substituted benzene ring the terminology is called orientation. So, what is the orientation of the group and how can we determine the orientation that depends on several factors? One of the factors is, of course electrophile is a must second factor is you are treating the benzene ring
the terminology is called orientation. So, what is the orientation of the group and how can we determine the orientation that depends on several factors? One of the factors is, of course electrophile is a must second factor is you are treating the benzene ring with a substitution. What sort of substitution is that? Is it an electron donating group, or it is an electron withdrawing group, say in this case methyl how should I know it is electron donating or electron withdrawing? Because the carbon-hydrogen bond each if I take elaborately the electronegativity difference of hydrogen and carbon, is there so electron pair forming the bond between hydrogen and carbon will be pushed towards the carbon atom. So, three such pushes will be there from three sides. So, what will happen to the carbon, the electron density will increase, and that electron density will again relay to the benzene ring
with a substitution. What sort of substitution is that? Is it an electron donating group, or it is an electron withdrawing group, say in this case methyl how should I know it is electron donating or electron withdrawing? Because the carbon-hydrogen bond each if I take elaborately the electronegativity difference of hydrogen and carbon, is there so electron pair forming the bond between hydrogen and carbon will be pushed towards the carbon atom. So, three such pushes will be there from three sides. So, what will happen to the carbon, the electron density will increase, and that electron density will again relay to the benzene ring . So, what will happen to the benzene ring
. So, what will happen to the benzene ring , so electron density gets increased compared to benzene
, so electron density gets increased compared to benzene , the toluene
, the toluene methyl substituted benzene-toluene
methyl substituted benzene-toluene . The electron density of the benzene ring
. The electron density of the benzene ring is getting increased by the inductive effect. Now, if I bring the nitro group that is coming from mixed acid, so what it will do in which position this NO2 group will enter that we can write by some structure like because of this push the carbon. This carbon’s electron density is increasing. So, this may be polarized in this side, so I said, write a structure clearly that this double bond is now being localized and you are ending up with the methyl group, and this double bond is now polarized over here, and this can delocalize on three sides and those delocalized structures are the resonating structure. What is this type of delocalization? We can again delocalize this negative charge on this side also, and by this way we are able to make CH3, CH3, CH3 and the negative charge is getting delocalized in this side, so where we are able to put the negative charge. If I put the substituted benzene ring
is getting increased by the inductive effect. Now, if I bring the nitro group that is coming from mixed acid, so what it will do in which position this NO2 group will enter that we can write by some structure like because of this push the carbon. This carbon’s electron density is increasing. So, this may be polarized in this side, so I said, write a structure clearly that this double bond is now being localized and you are ending up with the methyl group, and this double bond is now polarized over here, and this can delocalize on three sides and those delocalized structures are the resonating structure. What is this type of delocalization? We can again delocalize this negative charge on this side also, and by this way we are able to make CH3, CH3, CH3 and the negative charge is getting delocalized in this side, so where we are able to put the negative charge. If I put the substituted benzene ring , that is methyl group as the carbon number one, this carbon number two. This is three. This is four. This is five. This is six, so we are able to make negative charge or localize negative charge at two positions. Four position or sixth position and no other position, so what does it mean? The electrophile should enter either in two position or six position. They are equivalent or in four position, and these positions are called ortho, meta and para. So, in this way, ortho, meta and para. So, in one way we are able to see that electron donating groups are ortho, para orienting. What does it mean, that if in benzene ring
, that is methyl group as the carbon number one, this carbon number two. This is three. This is four. This is five. This is six, so we are able to make negative charge or localize negative charge at two positions. Four position or sixth position and no other position, so what does it mean? The electrophile should enter either in two position or six position. They are equivalent or in four position, and these positions are called ortho, meta and para. So, in this way, ortho, meta and para. So, in one way we are able to see that electron donating groups are ortho, para orienting. What does it mean, that if in benzene ring some group is there, I have taken one example that is methyl. It is not restricted to methyl any other chloro or any electron pushing group or tertiary butyl whatever, which can increase the electron density of the direct benzene rings carbon atom. Where the attachment is there, then it can relay the electron pair either in two position or four position or sixth position. Two and four para orienting. That means now it is very simple. If I start with toluene
some group is there, I have taken one example that is methyl. It is not restricted to methyl any other chloro or any electron pushing group or tertiary butyl whatever, which can increase the electron density of the direct benzene rings carbon atom. Where the attachment is there, then it can relay the electron pair either in two position or four position or sixth position. Two and four para orienting. That means now it is very simple. If I start with toluene , make one equivalent of the nitro group, then it will end up with ortho or para substituted benzene ring. So, this is para. This is ortho mixture of ortho and para substituted and amount of ortho and para depends on several factors. One of the interesting features is the steric factor and the reaction condition, but if we do further nitration, then what will happen? That means to this if I put one more nitro group NO2+, that means sufficient amount of mixed acid. Now there are two functional groups already present in the benzene ring
, make one equivalent of the nitro group, then it will end up with ortho or para substituted benzene ring. So, this is para. This is ortho mixture of ortho and para substituted and amount of ortho and para depends on several factors. One of the interesting features is the steric factor and the reaction condition, but if we do further nitration, then what will happen? That means to this if I put one more nitro group NO2+, that means sufficient amount of mixed acid. Now there are two functional groups already present in the benzene ring where the third electrophile will enter, though the electrophile is similar in nature, that is NO2+, so that NO2+ will not dictate. But the groups being present in the benzene ring
where the third electrophile will enter, though the electrophile is similar in nature, that is NO2+, so that NO2+ will not dictate. But the groups being present in the benzene ring that is methyl and NO2+. They will say which position will be suitable for that. That means electrophiles should enter in two substituted or 2-nitro toluene
that is methyl and NO2+. They will say which position will be suitable for that. That means electrophiles should enter in two substituted or 2-nitro toluene or 4-nitro toluene
or 4-nitro toluene in some positions. Let me show that what is that CH3 group is electron donating, nitro group is electron withdrawing very interesting phenomena. One is electron donating another is electron withdrawing. I have written that electron donating groups are ortho-para orienting. I can also write electron withdrawing groups are meta orienting, electron donating groups are ortho-para orienting, electron withdrawing groups are meta orienting. So, because of this methyl group, this position will be activated. This is the ortho. This position is also activated that is also ortho and with respect to the nitro group, the same position getting activated because nitro group is meta orienting, not ortho-para, similar fashion this nitro group is also activated because of the presence of this position by the nitro group, so both will help if I have sufficient amount of NO2+ to activate these two positions. Now, if I take this structure that is ortho nitro toluene
in some positions. Let me show that what is that CH3 group is electron donating, nitro group is electron withdrawing very interesting phenomena. One is electron donating another is electron withdrawing. I have written that electron donating groups are ortho-para orienting. I can also write electron withdrawing groups are meta orienting, electron donating groups are ortho-para orienting, electron withdrawing groups are meta orienting. So, because of this methyl group, this position will be activated. This is the ortho. This position is also activated that is also ortho and with respect to the nitro group, the same position getting activated because nitro group is meta orienting, not ortho-para, similar fashion this nitro group is also activated because of the presence of this position by the nitro group, so both will help if I have sufficient amount of NO2+ to activate these two positions. Now, if I take this structure that is ortho nitro toluene , what will happen methyl group is electron donating, so this will help to introduce in ortho and para position. This is the ortho position. This is the para position. Nitro group is meta orienting. This also helps to activate the same position that is meta with respect to this. This is the three position, one-three, so that is meta, so both the groups are helping to get the new electrophile or the same electrophile to come in the position marked as cross or right. So, what will be the end? End will be you end up with CH3, NO2, NO2, NO2 in both the cases, so you get a single product. That means nitration of toluene, with sufficient amount of mixed acid, should produce 2,4,6-trinito toluene
, what will happen methyl group is electron donating, so this will help to introduce in ortho and para position. This is the ortho position. This is the para position. Nitro group is meta orienting. This also helps to activate the same position that is meta with respect to this. This is the three position, one-three, so that is meta, so both the groups are helping to get the new electrophile or the same electrophile to come in the position marked as cross or right. So, what will be the end? End will be you end up with CH3, NO2, NO2, NO2 in both the cases, so you get a single product. That means nitration of toluene, with sufficient amount of mixed acid, should produce 2,4,6-trinito toluene or what I have written here as TNT
or what I have written here as TNT . So, in this case, two things we have discussed one is electron donating group helping to come to enter for the entry of the electrophile in ortho and para position and electron withdrawing group is helping to enter in the meta position. If they contradict, then it is very difficult if they help each other by means. One is the complement of other, then we get sufficient amount of one type of product and when the competition takes place be between the electron donating and the electron withdrawing normally electron donating groups getting little bit preference, but that is not the case in this case, so this is the way the nitro substituted benzene ring could be produced and, as I said then, by reduction, the nitro group could be converted to the amino group. So, if I start with a nitro substituted benzene ring that I stopped what will happen why this nitro benzene
. So, in this case, two things we have discussed one is electron donating group helping to come to enter for the entry of the electrophile in ortho and para position and electron withdrawing group is helping to enter in the meta position. If they contradict, then it is very difficult if they help each other by means. One is the complement of other, then we get sufficient amount of one type of product and when the competition takes place be between the electron donating and the electron withdrawing normally electron donating groups getting little bit preference, but that is not the case in this case, so this is the way the nitro substituted benzene ring could be produced and, as I said then, by reduction, the nitro group could be converted to the amino group. So, if I start with a nitro substituted benzene ring that I stopped what will happen why this nitro benzene will help the new electrophile to enter only in the meta position that we can show with the help of a resonating structure, because the nitro group is electron withdrawing group, it is pulling electron from the benzene ring
will help the new electrophile to enter only in the meta position that we can show with the help of a resonating structure, because the nitro group is electron withdrawing group, it is pulling electron from the benzene ring . So, what will happen? We get some sort of structure where we get the negative charge where you get the negative charge, getting delocalized on the nitro side and the positive charge is getting localized on the ortho position and this can have in a similar way the same sort of resonating structure. If I keep the nitro group intact, like this and play with the delocalized thing, what I see the positive charge getting delocalized from two to four position and by another way, this positive charge again could be delocalized in the sixth position also, so what is happening here? This is the ortho position, two position. This is the sixth position, also ortho position. This is the para position, so this three position that is ortho and para - is positively charged. So, if you bring an electrophile which is positively charged, it will not enter there, where is the second possibility that will enter exclusively to the meta position. So that is the reason if I start with nitro benzene
. So, what will happen? We get some sort of structure where we get the negative charge where you get the negative charge, getting delocalized on the nitro side and the positive charge is getting localized on the ortho position and this can have in a similar way the same sort of resonating structure. If I keep the nitro group intact, like this and play with the delocalized thing, what I see the positive charge getting delocalized from two to four position and by another way, this positive charge again could be delocalized in the sixth position also, so what is happening here? This is the ortho position, two position. This is the sixth position, also ortho position. This is the para position, so this three position that is ortho and para - is positively charged. So, if you bring an electrophile which is positively charged, it will not enter there, where is the second possibility that will enter exclusively to the meta position. So that is the reason if I start with nitro benzene , I will end up with 2, 4, 6-tri nitro benzene.
, I will end up with 2, 4, 6-tri nitro benzene.
 What is that? nitro group will deactivate the benzene ring
What is that? nitro group will deactivate the benzene ring will pull electron help the second nitro group to come in the meta position. So, I am writing another NO2 and that nitro group will be entered by this pi complex, sigma complex that same methodology. The three position, and now very interesting thing - has happened because this nitro group will activate this meta position and the other nitro group also will activate the same meta position. So if I do a further nitration with NO2+, I will end up with NO2, NO2, NO2 that means. Starting from these, we are able to make 1, 3, 5-tri nitro benzene
will pull electron help the second nitro group to come in the meta position. So, I am writing another NO2 and that nitro group will be entered by this pi complex, sigma complex that same methodology. The three position, and now very interesting thing - has happened because this nitro group will activate this meta position and the other nitro group also will activate the same meta position. So if I do a further nitration with NO2+, I will end up with NO2, NO2, NO2 that means. Starting from these, we are able to make 1, 3, 5-tri nitro benzene , so benzene on nitration gives nitro benzene
, so benzene on nitration gives nitro benzene , nitrobenzene
, nitrobenzene on further nitration can give 1, 3-dinitro benzene, 1, 3-dinitrobenzene on further nitration should give 1, 3, 5-tri nitro benzene
on further nitration can give 1, 3-dinitro benzene, 1, 3-dinitrobenzene on further nitration should give 1, 3, 5-tri nitro benzene , so this is one way out to make carbon-nitrogen bonds by the help of electrophile obtained from simple mixed acids, like NO2+ that electrophile now should enter the benzene ring
, so this is one way out to make carbon-nitrogen bonds by the help of electrophile obtained from simple mixed acids, like NO2+ that electrophile now should enter the benzene ring in three positions and when all this thing is done, if I do further nitration, then question comes where it will go actually now there is no vacant position available over there, and this will not be a good way to proceed further, because this will be an awkward mixture of several things will take place, so the there is no activation or help by the group already present in the benzene ring
in three positions and when all this thing is done, if I do further nitration, then question comes where it will go actually now there is no vacant position available over there, and this will not be a good way to proceed further, because this will be an awkward mixture of several things will take place, so the there is no activation or help by the group already present in the benzene ring . So this is the general rule for orientation. When one group is there how the second group is coming, if the second group is there if they are complementary to each other, that helps, if not, then of course, the electron donating groups gets little bit preference if they complement, then it is a very nice way to make the substituted compound of the products, so this is one way to make the benzene very easily another thing I should say that nitro benzene
. So this is the general rule for orientation. When one group is there how the second group is coming, if the second group is there if they are complementary to each other, that helps, if not, then of course, the electron donating groups gets little bit preference if they complement, then it is a very nice way to make the substituted compound of the products, so this is one way to make the benzene very easily another thing I should say that nitro benzene , to substituted nitro benzene or more positions in the benzene ring
, to substituted nitro benzene or more positions in the benzene ring more nitro groups could be attached and, as I told you, oxidation-reduction steps are very simple. You can convert nitro to the amine very easily, and this amine, that is this aniline
more nitro groups could be attached and, as I told you, oxidation-reduction steps are very simple. You can convert nitro to the amine very easily, and this amine, that is this aniline , like the methyl amine I started with, is a very important starting material, because from here you can make many interesting carbon-nitrogen compounds. You know one of the reactions is treatment of aniline
, like the methyl amine I started with, is a very important starting material, because from here you can make many interesting carbon-nitrogen compounds. You know one of the reactions is treatment of aniline , with nitrous acid (HNO2) at lower temperature, say 0 degree centigrade. What will happen? If I treat aniline
, with nitrous acid (HNO2) at lower temperature, say 0 degree centigrade. What will happen? If I treat aniline with nitrous acid (HNO2) as the source of nitrous acid (HNO2), sodium nitrite (NaNO2) and hydrochloric acid (HCl)? If I mix them together, it will form nitrous acid, HNO2 and NaCl sodium chloride and at lower temperature this amine, when treated with nitrous acid (HNO2), at ice cold temperature. Then we see a very interesting thing, that is, I should say, a species like this N2+Cl- what you call this type of compound, because in this case I can see that two nitrogen being attached and the counter ion is the chloride as hydrochloric acid being used, so this type of compound will be called di means two, aza means nitrogen, so diaza compound or diazonium salt, and this diazonium salt is very interesting if 2-alkaline beta-naphthol
with nitrous acid (HNO2) as the source of nitrous acid (HNO2), sodium nitrite (NaNO2) and hydrochloric acid (HCl)? If I mix them together, it will form nitrous acid, HNO2 and NaCl sodium chloride and at lower temperature this amine, when treated with nitrous acid (HNO2), at ice cold temperature. Then we see a very interesting thing, that is, I should say, a species like this N2+Cl- what you call this type of compound, because in this case I can see that two nitrogen being attached and the counter ion is the chloride as hydrochloric acid being used, so this type of compound will be called di means two, aza means nitrogen, so diaza compound or diazonium salt, and this diazonium salt is very interesting if 2-alkaline beta-naphthol . I am writing this - is the benzene ring
. I am writing this - is the benzene ring . This is naphthalene
. This is naphthalene . This is the one position. This is the two position in two position if there be an OH group, this compound is called beta-naphthol or two-naphthol
. This is the one position. This is the two position in two position if there be an OH group, this compound is called beta-naphthol or two-naphthol , and this beta-naphthol
, and this beta-naphthol , sodium hydroxide (NaOH) or potassium hydroxide (KOH) solution very interesting feature: two alkaline beta-naphthol
, sodium hydroxide (NaOH) or potassium hydroxide (KOH) solution very interesting feature: two alkaline beta-naphthol , either in sodium hydroxide (NaOH) or potassium hydroxide (KOH). If we add this diazotized or diaza compound diazotized aromatic compounds very simple cases - diazonium salt, like this, will get a very nice, beautiful red color dye. What is that? A long delocalized, a naphthalene ring
, either in sodium hydroxide (NaOH) or potassium hydroxide (KOH). If we add this diazotized or diaza compound diazotized aromatic compounds very simple cases - diazonium salt, like this, will get a very nice, beautiful red color dye. What is that? A long delocalized, a naphthalene ring getting delocalized to the other benzene ring
getting delocalized to the other benzene ring through the nitrogen-nitrogen double bond. So long delocalized electron clouds are being formed and because of that, the compound is bound to be deep red in color. So, a red dye is formed this way. So, what I should say is that aromatic amine could be detected through the formation of a red dye, and this is a very good confirmative test for aryl amine, especially aniline
through the nitrogen-nitrogen double bond. So long delocalized electron clouds are being formed and because of that, the compound is bound to be deep red in color. So, a red dye is formed this way. So, what I should say is that aromatic amine could be detected through the formation of a red dye, and this is a very good confirmative test for aryl amine, especially aniline when being diazotized and remember 2-alkaline beta-naphthol
when being diazotized and remember 2-alkaline beta-naphthol  we’re adding the diazotized compound, not the other way around under cold condition, then it forms a red dye, and that red dye is very, very characteristic why this is more color or deep in color. Why? It is this that could be explained by a very simple phenomenon. There is a very general rule if there is a long, conjugated polyene system, as in this case one benzene ring, the second benzene ring that is naphthalene
we’re adding the diazotized compound, not the other way around under cold condition, then it forms a red dye, and that red dye is very, very characteristic why this is more color or deep in color. Why? It is this that could be explained by a very simple phenomenon. There is a very general rule if there is a long, conjugated polyene system, as in this case one benzene ring, the second benzene ring that is naphthalene . The third one is also connected through the nitrogen-nitrogen double bond. So, it is a long-delocalized system. So, when the electron cloud is getting spread over long or many atoms, then what will happen if you think in this way, E = h𝜈 and when a more conjugated polyene being taken that energy to take it from the ground to the excited state will be much less if there be more conjugation than when it is an isolated double bond or simple benzene
. The third one is also connected through the nitrogen-nitrogen double bond. So, it is a long-delocalized system. So, when the electron cloud is getting spread over long or many atoms, then what will happen if you think in this way, E = h𝜈 and when a more conjugated polyene being taken that energy to take it from the ground to the excited state will be much less if there be more conjugation than when it is an isolated double bond or simple benzene being like this, what does it mean if the energy needed is less h is planck’s constant also, will be less if is less. What is the lambda reverse of frequency that is , so lambda will be more so what is happening in other way, a simple butadiene
being like this, what does it mean if the energy needed is less h is planck’s constant also, will be less if is less. What is the lambda reverse of frequency that is , so lambda will be more so what is happening in other way, a simple butadiene and you take carotene
and you take carotene , tetra substituted butadiene or long polyene. The long polyene or carotene
, tetra substituted butadiene or long polyene. The long polyene or carotene , that is C40, is color red color. That is being present in tomato and carrots, why those are red or orange in color, but butadiene
, that is C40, is color red color. That is being present in tomato and carrots, why those are red or orange in color, but butadiene is colorless? The answer is because it is more conjugated, more conjugated means the more delocalized the electron is taking place, and in that case the energy necessary to take needed to take from the ground to the excited state, which is very important phenomena for the color thing is less so, as energy is less frequency is less frequency is less means wavelength more. We can see 400 to 800 nanometer 200 to 400 is the ultraviolet region, so the compounds are getting colored. So, this is another general technique by the help of carbon, nitrogen chemistry, one can make a colorless to color by putting more conjugation, especially with some chromophoric group or auxo chromic group, that also help. So, this is an offshoot or the bonus of carbon-nitrogen chemistry. So, what is the advantage of that advantage is tremendous suppose you have converted a diazo compound. Ah, the way I said, take the aniline
is colorless? The answer is because it is more conjugated, more conjugated means the more delocalized the electron is taking place, and in that case the energy necessary to take needed to take from the ground to the excited state, which is very important phenomena for the color thing is less so, as energy is less frequency is less frequency is less means wavelength more. We can see 400 to 800 nanometer 200 to 400 is the ultraviolet region, so the compounds are getting colored. So, this is another general technique by the help of carbon, nitrogen chemistry, one can make a colorless to color by putting more conjugation, especially with some chromophoric group or auxo chromic group, that also help. So, this is an offshoot or the bonus of carbon-nitrogen chemistry. So, what is the advantage of that advantage is tremendous suppose you have converted a diazo compound. Ah, the way I said, take the aniline very simple case: diazotize it with nitrous acid (HNO2) and get the diazo compound, and now you want to remove the diazo compound and put some substitution, because now the N2+ is directly attached over there. You can do it in a very nice way. There is a term I have written here. CuX and HX, Cuprous halide or X may be many things, cyanide, chloride, etc. So, what will happen? The whole thing this N2+Cl-, will go and the X will be inserted straight over there. That means from this diazo compound you can get the cyano compound, and in that way, you can make many, many interesting aromatic compounds and will take some more examples later, but this type of reaction was first studied by Sandmeyer, so this is known as Sandmeyer reaction, so one of the way to make the several substituted aromatic compounds is by the help of carbon-nitrogen compound through this diazonium salt and then with the Sandmeyer reaction, where CuX and HX, be the reagent X is chloride, bromide, cyanide, etc. Ok, so I started with amine first aliphatic, then aromatic question comes not only aliphatic and aromatic where the direct carbon-nitrogen bonds is a single bond. There may be a carbon-nitrogen bond as a triple bond or a double bond. Let me write down a very simple case. If I write this type of structure where I am putting the substituent as CH3, CH3 double bond. Of course, I have to satisfy the valency as NH, here the case is different. That is not a single bond, but there is a carbon-nitrogen double bond is there, and these types of compounds are also very interesting. These are called not amine but imine, and they could be prepared the way I started that you can break this molecule and get some clue as synthon or synthetic equivalent and then ending up with the starting material as simple as acetone
very simple case: diazotize it with nitrous acid (HNO2) and get the diazo compound, and now you want to remove the diazo compound and put some substitution, because now the N2+ is directly attached over there. You can do it in a very nice way. There is a term I have written here. CuX and HX, Cuprous halide or X may be many things, cyanide, chloride, etc. So, what will happen? The whole thing this N2+Cl-, will go and the X will be inserted straight over there. That means from this diazo compound you can get the cyano compound, and in that way, you can make many, many interesting aromatic compounds and will take some more examples later, but this type of reaction was first studied by Sandmeyer, so this is known as Sandmeyer reaction, so one of the way to make the several substituted aromatic compounds is by the help of carbon-nitrogen compound through this diazonium salt and then with the Sandmeyer reaction, where CuX and HX, be the reagent X is chloride, bromide, cyanide, etc. Ok, so I started with amine first aliphatic, then aromatic question comes not only aliphatic and aromatic where the direct carbon-nitrogen bonds is a single bond. There may be a carbon-nitrogen bond as a triple bond or a double bond. Let me write down a very simple case. If I write this type of structure where I am putting the substituent as CH3, CH3 double bond. Of course, I have to satisfy the valency as NH, here the case is different. That is not a single bond, but there is a carbon-nitrogen double bond is there, and these types of compounds are also very interesting. These are called not amine but imine, and they could be prepared the way I started that you can break this molecule and get some clue as synthon or synthetic equivalent and then ending up with the starting material as simple as acetone and say, ammonia (NH3) or derivative of that so, if you treat acetone
and say, ammonia (NH3) or derivative of that so, if you treat acetone now, the starting materials are amine and acetone
now, the starting materials are amine and acetone together. What will happen? This is electron rich. This is the electron deficient. How did I know because carbonyl groups, carbon is positively charged, oxygen is negatively charged. There will be a polarization possible because oxygen is more electronegative compared to carbon, so electron pair forming the bond will be shifted more towards oxygen, so making the carbon as electro positive. So, the amine will go over there very easily, and, in that process, you get CH3CCH3O- and this side is NHH2 and one of the hydrogen - if I put it in this way, will be picked up by the O-, so you end up with CH3CCH3, and this is now OHNHH. So, this is the way the amine group and the OH group has been introduced to the acetone moiety, which comes from carbonyl and NH2 is coming from ammonia. Then what happens? This type of compound is very interesting because, simply by heating, it loses water, it loses water means how the one of this nitrogen hydrogen bond leaves, at the same time, the OH also leaving the system so H and OH leaving the system at the same time. What type of reaction is this one? This is called an elimination reaction, elimination reaction, so in elimination reaction what is happening? You get (CH3)2C=NH, so one simple way to make the amine from ammonia in this case or substituted ammonia also, you can take is very simple reactions where a nucleophile is attacking carbonyl carbon. The nucleophile is the ammonia or substituted ammonia, and then an elimination reaction takes place where the hydrogen and hydroxyl group is leaving at the same time, this type of elimination is called beta-elimination and one of the interesting features is anti-group means hydrogen and which this is not a very complicated one, this is a very simple case, hydrogen and OH leaving the system at the same time. So, this is a beta elimination reaction by which we are able to make the imine so carbon-nitrogen single bond we have discussed in detail, carbon-nitrogen double bonds also are very important and from there many interesting compounds could be prepared.
together. What will happen? This is electron rich. This is the electron deficient. How did I know because carbonyl groups, carbon is positively charged, oxygen is negatively charged. There will be a polarization possible because oxygen is more electronegative compared to carbon, so electron pair forming the bond will be shifted more towards oxygen, so making the carbon as electro positive. So, the amine will go over there very easily, and, in that process, you get CH3CCH3O- and this side is NHH2 and one of the hydrogen - if I put it in this way, will be picked up by the O-, so you end up with CH3CCH3, and this is now OHNHH. So, this is the way the amine group and the OH group has been introduced to the acetone moiety, which comes from carbonyl and NH2 is coming from ammonia. Then what happens? This type of compound is very interesting because, simply by heating, it loses water, it loses water means how the one of this nitrogen hydrogen bond leaves, at the same time, the OH also leaving the system so H and OH leaving the system at the same time. What type of reaction is this one? This is called an elimination reaction, elimination reaction, so in elimination reaction what is happening? You get (CH3)2C=NH, so one simple way to make the amine from ammonia in this case or substituted ammonia also, you can take is very simple reactions where a nucleophile is attacking carbonyl carbon. The nucleophile is the ammonia or substituted ammonia, and then an elimination reaction takes place where the hydrogen and hydroxyl group is leaving at the same time, this type of elimination is called beta-elimination and one of the interesting features is anti-group means hydrogen and which this is not a very complicated one, this is a very simple case, hydrogen and OH leaving the system at the same time. So, this is a beta elimination reaction by which we are able to make the imine so carbon-nitrogen single bond we have discussed in detail, carbon-nitrogen double bonds also are very important and from there many interesting compounds could be prepared.
 One interesting compound I can say that one very reactive compound could be prepared by this way is carbon double bond N and if we put some substituents over, there could be oxidized, and this oxidation is very interesting to make an oxide ring nitrogen, oxygen, carbon containing compound. These are also very important and very explosive nature compounds. So, this is another field which is coming originally from nitrogen, containing organic compounds. Ok, third thing: what I left is a carbon-nitrogen triple bonded compound. That means how to make carbon-nitrogen triple bonded compound, and what is the use of that? I can take a very simple example lets satisfy the valency of carbon RC triple bond N, usually in organic chemistry. These are not called cyanide. These are nitriles. So, these nitriles, when hydrolyzed treated with water in presence of little bit acid or alkali what will happen, that means RCN treated with H2O in presence of H+ or OH- what will be the product? Nucleophile definitely will attack this carbon and this carbon-ntrogen bond out of the triple bond one will be polarized towards the nitrogen atom. So, what I see RCOHH. This will be the positively charged and then one of this bond has shifted. So, this will be the minus over here. That is the negative recharge. This sort of thing will take place, so obviously this is not a very stable species. So, what will happen? The hydrogen could be picked up by this N-, so it ends up with RCOH and this side will be double bond NH, RC=NH and OH, this type of feature again we have ended up with a triple bonded to a double bonded thing, but if I write that this can be stabilized in a way like this, the electron cloud is shifting in from one to the other and a very interesting phenomena. And here I have written the reversible side, not electron delocalization. This is a phenomenon where the proton is shifting from this position to that position, proton delocalization is another way to call it is tautomerism, so tautomerism is taking place and we have ended up with a compound is RCONH2. What is that compound? This is the amide. So, from nitrile we have ended up with an amide how by hydrolysis, what sort of hydrolysis, either acid catalyzed, or base catalyzed what is happening to RC triple bond N. It has been converted to RCONH2 so amide being formed from the nitrile and amide is a very important structural features from here you can make polyamide, then many other polymeric materials which are used in everyday life. So, polyamide is a very important feature where the starting material is coming from the nitrile. So, carbon-nitrogen triple bond is also being mentioned over here: carbon-nitrogen, single bond, carbon-nitrogen, double bond and carbon-nitrogen triple bond, and that could be converted to RCO and NH2. Sometimes I ask my students that I have done this reaction from alkyl cyanide to the amide by hydrolysis in this way and let us take an example suppose I have given you acetamide CH3CONH2
One interesting compound I can say that one very reactive compound could be prepared by this way is carbon double bond N and if we put some substituents over, there could be oxidized, and this oxidation is very interesting to make an oxide ring nitrogen, oxygen, carbon containing compound. These are also very important and very explosive nature compounds. So, this is another field which is coming originally from nitrogen, containing organic compounds. Ok, third thing: what I left is a carbon-nitrogen triple bonded compound. That means how to make carbon-nitrogen triple bonded compound, and what is the use of that? I can take a very simple example lets satisfy the valency of carbon RC triple bond N, usually in organic chemistry. These are not called cyanide. These are nitriles. So, these nitriles, when hydrolyzed treated with water in presence of little bit acid or alkali what will happen, that means RCN treated with H2O in presence of H+ or OH- what will be the product? Nucleophile definitely will attack this carbon and this carbon-ntrogen bond out of the triple bond one will be polarized towards the nitrogen atom. So, what I see RCOHH. This will be the positively charged and then one of this bond has shifted. So, this will be the minus over here. That is the negative recharge. This sort of thing will take place, so obviously this is not a very stable species. So, what will happen? The hydrogen could be picked up by this N-, so it ends up with RCOH and this side will be double bond NH, RC=NH and OH, this type of feature again we have ended up with a triple bonded to a double bonded thing, but if I write that this can be stabilized in a way like this, the electron cloud is shifting in from one to the other and a very interesting phenomena. And here I have written the reversible side, not electron delocalization. This is a phenomenon where the proton is shifting from this position to that position, proton delocalization is another way to call it is tautomerism, so tautomerism is taking place and we have ended up with a compound is RCONH2. What is that compound? This is the amide. So, from nitrile we have ended up with an amide how by hydrolysis, what sort of hydrolysis, either acid catalyzed, or base catalyzed what is happening to RC triple bond N. It has been converted to RCONH2 so amide being formed from the nitrile and amide is a very important structural features from here you can make polyamide, then many other polymeric materials which are used in everyday life. So, polyamide is a very important feature where the starting material is coming from the nitrile. So, carbon-nitrogen triple bond is also being mentioned over here: carbon-nitrogen, single bond, carbon-nitrogen, double bond and carbon-nitrogen triple bond, and that could be converted to RCO and NH2. Sometimes I ask my students that I have done this reaction from alkyl cyanide to the amide by hydrolysis in this way and let us take an example suppose I have given you acetamide CH3CONH2 , and I want to create acetonitrile that is CH3CN
, and I want to create acetonitrile that is CH3CN  how to do that? People get confused, but a very general rule is if from nitrile to amide you do the hydrolysis means breaking with water, so obviously from amide to nitrile what will you do? Dehydration and what are dehydrating agents? There are many phosphorus pentoxides (P2O5), sulphuric acid (H2SO4), which can take care of the water. So as simple as that, how will it proceed the same phenomena because it has, it is having an alpha hydrogen atom. It will undergo some sort of tautomerism. The way I have shown in the previous case - and then this will go, this will be eliminated, the hydrogen and OH will be eliminated, and you end up with CH3C triple bond N, so CH3C triple bond N that is nitrile is coming from the amide by dehydration and nitrile getting hydrolyzed to the amide by hydrolysis, so this is another trick to make the carbon-nitrogen triple bond and use it for the preparation of important compounds like amides, polyamides and all other important feature. I will tell another interesting feature of nitrogen containing organic compounds that I did not mention much. That is if I write a benzene ring
how to do that? People get confused, but a very general rule is if from nitrile to amide you do the hydrolysis means breaking with water, so obviously from amide to nitrile what will you do? Dehydration and what are dehydrating agents? There are many phosphorus pentoxides (P2O5), sulphuric acid (H2SO4), which can take care of the water. So as simple as that, how will it proceed the same phenomena because it has, it is having an alpha hydrogen atom. It will undergo some sort of tautomerism. The way I have shown in the previous case - and then this will go, this will be eliminated, the hydrogen and OH will be eliminated, and you end up with CH3C triple bond N, so CH3C triple bond N that is nitrile is coming from the amide by dehydration and nitrile getting hydrolyzed to the amide by hydrolysis, so this is another trick to make the carbon-nitrogen triple bond and use it for the preparation of important compounds like amides, polyamides and all other important feature. I will tell another interesting feature of nitrogen containing organic compounds that I did not mention much. That is if I write a benzene ring now it is very clear and remove one of the carbons by the nitrogen atom. Then there are five hydrogen atoms now directly attached to the carbon and one of the CH being replaced by nitrogen. So, this type of compound is carbon-nitrogen compounds, but I should say most precisely: this is the heterocyclic aromatic compound. Why heterocyclic? Because heteroatoms are present, why cyclic? Because if I start from the one end, I am ending up at the same atom and it is aromatic because it is just like benzene rings, but one of the carbons being replaced by nitrogen. So, it follows the Huckle’s rule. That is planar conjugated cyclic compound having 4n+2 pi electrons, so all those rules are being followed. So, this is a heterocyclic aromatic compound. Likewise, if I write another structure where it is the five-membered ring where one of the member is the nitrogen and then the hydrogen atoms are there each having one? And if I look at this molecule, have I seen this type of molecule anywhere? The answer is yes, this is a heterocyclic compound having one nitrogen in the ring, and this compound is also aromatic in nature. So, we are able to get two types of aromatic heterocyclic compounds. What are those two types? One is a five-member. Another is a six-member and in each case, as we are, we have focused our attention on carbon-nitrogen compounds. So, one of the ring members is nitrogen, one is called pyrrole
now it is very clear and remove one of the carbons by the nitrogen atom. Then there are five hydrogen atoms now directly attached to the carbon and one of the CH being replaced by nitrogen. So, this type of compound is carbon-nitrogen compounds, but I should say most precisely: this is the heterocyclic aromatic compound. Why heterocyclic? Because heteroatoms are present, why cyclic? Because if I start from the one end, I am ending up at the same atom and it is aromatic because it is just like benzene rings, but one of the carbons being replaced by nitrogen. So, it follows the Huckle’s rule. That is planar conjugated cyclic compound having 4n+2 pi electrons, so all those rules are being followed. So, this is a heterocyclic aromatic compound. Likewise, if I write another structure where it is the five-membered ring where one of the member is the nitrogen and then the hydrogen atoms are there each having one? And if I look at this molecule, have I seen this type of molecule anywhere? The answer is yes, this is a heterocyclic compound having one nitrogen in the ring, and this compound is also aromatic in nature. So, we are able to get two types of aromatic heterocyclic compounds. What are those two types? One is a five-member. Another is a six-member and in each case, as we are, we have focused our attention on carbon-nitrogen compounds. So, one of the ring members is nitrogen, one is called pyrrole , another is called pyridine
, another is called pyridine , very common base. Very common base how did I say, because if I look at the number of electrons over there, I can see this nitrogen lone pair is very easily available, which it can donate to any acidic compound. So, this is a basic compound or pyridine
, very common base. Very common base how did I say, because if I look at the number of electrons over there, I can see this nitrogen lone pair is very easily available, which it can donate to any acidic compound. So, this is a basic compound or pyridine is a very good, solvent and also very good base, which is a heterocyclic compound having nitrogen in the benzene ring
is a very good, solvent and also very good base, which is a heterocyclic compound having nitrogen in the benzene ring . One of the benzene rings carbons is being replaced by N, and this is basic in nature because it fulfills the aromaticity very easily. But if I ask you, what is the nature of these five-member nitrogen containing compounds? Is it aromatic? Yes, it is aromatic how? Because I am taking the two-nitrogen electron + 4 from the conjugated system and why it is conjugated because double-single-double-single though two singles are coming. But it is a delocalized thing, so Huckle’s rule, if we recapture or recapitulate it will be planar conjugated cyclic compound having 4n+2 pi electron, where n is 1 in this case, that is 4*1 = 4+2 = 6 pi electron and exactly it Is fitting that it is planar all are sp2 hybridized carbon. This is nitrogen and 2+2+2 that is 6, 2 from two pairs of carbon and 2 from the nitrogen atom, and so six electrons’ rules are also being followed. And, this is now fully aromatic in nature, but what is happening by this process? The nitrogen in this case six membered - is available. The electron pair on nitrogen is available to other substrate. So that is why I have written a term base. Pyridine
. One of the benzene rings carbons is being replaced by N, and this is basic in nature because it fulfills the aromaticity very easily. But if I ask you, what is the nature of these five-member nitrogen containing compounds? Is it aromatic? Yes, it is aromatic how? Because I am taking the two-nitrogen electron + 4 from the conjugated system and why it is conjugated because double-single-double-single though two singles are coming. But it is a delocalized thing, so Huckle’s rule, if we recapture or recapitulate it will be planar conjugated cyclic compound having 4n+2 pi electron, where n is 1 in this case, that is 4*1 = 4+2 = 6 pi electron and exactly it Is fitting that it is planar all are sp2 hybridized carbon. This is nitrogen and 2+2+2 that is 6, 2 from two pairs of carbon and 2 from the nitrogen atom, and so six electrons’ rules are also being followed. And, this is now fully aromatic in nature, but what is happening by this process? The nitrogen in this case six membered - is available. The electron pair on nitrogen is available to other substrate. So that is why I have written a term base. Pyridine is a base, but in case of pyrrole
is a base, but in case of pyrrole I cannot write that term. Why not? The reason being the lone pair of nitrogen is now being taken the aromaticity gaining five-member unit, so it is not available. So, what happens it acts as an acidity. So, there is a very general question. Nice question is asked: how come five-member nitrogen containing delocalized compound that is Pyrrole
I cannot write that term. Why not? The reason being the lone pair of nitrogen is now being taken the aromaticity gaining five-member unit, so it is not available. So, what happens it acts as an acidity. So, there is a very general question. Nice question is asked: how come five-member nitrogen containing delocalized compound that is Pyrrole is acidic in nature and pyridine
is acidic in nature and pyridine , which is a six membered nitrogen containing aromatic compound is alkaline or basic in nature? Answer is the electron density on the nitrogen of the pyridine
, which is a six membered nitrogen containing aromatic compound is alkaline or basic in nature? Answer is the electron density on the nitrogen of the pyridine is available to be donated, but that electron density of the pyrrole
is available to be donated, but that electron density of the pyrrole unit, which is a five membered where it’s the two electrons are being given to the benzene thing or the five membered ring to gain the aromaticity is not available, so it is electron deficient in that case, so it as per Lewis theory. An electron donor is a good base and electron acceptor is an acid, so pyrrole
unit, which is a five membered where it’s the two electrons are being given to the benzene thing or the five membered ring to gain the aromaticity is not available, so it is electron deficient in that case, so it as per Lewis theory. An electron donor is a good base and electron acceptor is an acid, so pyrrole is acidic in that way. Pyridine
is acidic in that way. Pyridine is basic in that way if we consider. So, this is another interesting phenomenon where we see the carbon-nitrogen compound are not only on the side chain or directly attached to the alkyl group directly attached to the aromatic group. But it may be a part of the benzene ring
is basic in that way if we consider. So, this is another interesting phenomenon where we see the carbon-nitrogen compound are not only on the side chain or directly attached to the alkyl group directly attached to the aromatic group. But it may be a part of the benzene ring or five membered or seven membered or in higher series. Also, nitrogen’s role is tremendous in biological system.
or five membered or seven membered or in higher series. Also, nitrogen’s role is tremendous in biological system.
 I did not say anything about another important class of carbon-nitrogen compound. People say that antibiotics are, as I told you, beta-lactam
I did not say anything about another important class of carbon-nitrogen compound. People say that antibiotics are, as I told you, beta-lactam related compounds, not one class. There are many classes of antibiotics. Beta-lactam
related compounds, not one class. There are many classes of antibiotics. Beta-lactam is very important, which is nothing but carbon-nitrogen containing compound second portion came is the amino acid, which is the building block for protein, peptide, polypeptide third thing is: if we take the pyrrole
is very important, which is nothing but carbon-nitrogen containing compound second portion came is the amino acid, which is the building block for protein, peptide, polypeptide third thing is: if we take the pyrrole units together, four pyrrole
units together, four pyrrole units being connected by carbon atoms. If I write it in that way, it will be N, N just I have written arbitrarily one structure where four pyrrole
units being connected by carbon atoms. If I write it in that way, it will be N, N just I have written arbitrarily one structure where four pyrrole units are there in between. Normally there are one carbon or substituted one just to fill the importance of this class of compounds where four pyrrole
units are there in between. Normally there are one carbon or substituted one just to fill the importance of this class of compounds where four pyrrole units are on four sides and each pyrrole
units are on four sides and each pyrrole unit in the two position - and this will be three, four, five position: two position and five position being connected by another carbon atom - or it may be directly connected to another pyrrole
unit in the two position - and this will be three, four, five position: two position and five position being connected by another carbon atom - or it may be directly connected to another pyrrole unit, so that sort of structural features makes a cavity, and this cavity is very interesting to fit many metal ions and those metal ions. Giving that type of compounds very interesting feature very interesting biological activities very interesting colors, and if I ask you, have you seen this type of four pyrrole
unit, so that sort of structural features makes a cavity, and this cavity is very interesting to fit many metal ions and those metal ions. Giving that type of compounds very interesting feature very interesting biological activities very interesting colors, and if I ask you, have you seen this type of four pyrrole units being connected through each one, carbon atom or substituted carbon atom and making a ring macro cyclic ring of that way? One interesting thing is that types of compounds in general are called porphyrin
units being connected through each one, carbon atom or substituted carbon atom and making a ring macro cyclic ring of that way? One interesting thing is that types of compounds in general are called porphyrin or, I should say, pyrroles (4) four units together and this type of porphyrin
or, I should say, pyrroles (4) four units together and this type of porphyrin in natural products we see is very much present in two three interesting compounds: what we do for everyday life. What is that, why is blood red in color? Everybody knows blood contains hemoglobin heme is that poly pyrrole unit where a metal ion is being there. So, heme is iron is the metal ion like why the green leaves are there? Why are the leaves green in color? Answer is very simple, that is the chlorophyll and in chlorophyll the basic unit structural units is the porphyrin
in natural products we see is very much present in two three interesting compounds: what we do for everyday life. What is that, why is blood red in color? Everybody knows blood contains hemoglobin heme is that poly pyrrole unit where a metal ion is being there. So, heme is iron is the metal ion like why the green leaves are there? Why are the leaves green in color? Answer is very simple, that is the chlorophyll and in chlorophyll the basic unit structural units is the porphyrin , and this porphyrin
, and this porphyrin means that four pyrrole unit being connected in two and five position through one carbon atom each so that makes a cavity and within cavity the calcium of magnesium or many ions could be fitted and different types of color, biologically active, pigments and medicines are being produced. One simple example is heme. Globin is the protein part. The second example is chlorophyll that is green in color and third example is cyanocobalamin, which is a little bit complicated in structure, but basic unity is poly pyrrole. That is present in vitamin B12. So, vitamin B12 is also a bicomplex, very important member. So, these are the important features of the carbon-nitrogen compound in everyday life or in medicinal things, or in other ways. I should say one more thing to end up: today’s, talk that other than antibiotics other than amino acids, protein, peptides other than the amides or heterocyclic compounds like pyrrole
means that four pyrrole unit being connected in two and five position through one carbon atom each so that makes a cavity and within cavity the calcium of magnesium or many ions could be fitted and different types of color, biologically active, pigments and medicines are being produced. One simple example is heme. Globin is the protein part. The second example is chlorophyll that is green in color and third example is cyanocobalamin, which is a little bit complicated in structure, but basic unity is poly pyrrole. That is present in vitamin B12. So, vitamin B12 is also a bicomplex, very important member. So, these are the important features of the carbon-nitrogen compound in everyday life or in medicinal things, or in other ways. I should say one more thing to end up: today’s, talk that other than antibiotics other than amino acids, protein, peptides other than the amides or heterocyclic compounds like pyrrole , quinoline
, quinoline , pyridine
, pyridine , pyrimidine
, pyrimidine . Those are the building blocks of life DNA, RNA. Another important nitrogen containing organic compounds are alkaloids, so alkaloids are, by definition is alkaline compound, alkaline mostly in nature obtained from natural sources having nitrogen will have to have carbon and have some medicinal value. So medicinally important nitrogen containing compounds are the alkaloids which are alkaline in nature. So that thing I did not mention. If I ask you, can you name some alkaloids? Yes, quinine
. Those are the building blocks of life DNA, RNA. Another important nitrogen containing organic compounds are alkaloids, so alkaloids are, by definition is alkaline compound, alkaline mostly in nature obtained from natural sources having nitrogen will have to have carbon and have some medicinal value. So medicinally important nitrogen containing compounds are the alkaloids which are alkaline in nature. So that thing I did not mention. If I ask you, can you name some alkaloids? Yes, quinine , nicotine
, nicotine , pyrimidine
, pyrimidine ? There are many alkaloids and some of the narcotics also have the carbon-nitrogen unit being present.
? There are many alkaloids and some of the narcotics also have the carbon-nitrogen unit being present.
 So, to sum up, we have discussed about the importance of carbon nitrogen compounds and how to prepare them specially for aromatic system, through the help of the nitro group and then by reduction and to convert the nitro to amine is very simple reduction and amine to diazo not much difficult, that is sodium nitrite (NaNO2), hydrochloric acid (HCl), diazotization and then by using Sandmeyer reaction, almost any functionality could be introduced where the diazo group will go, and the new group will come and the use of those compounds in the functional group transformation. I will continue with other aspects of the carbon-nitrogen compounds next time. Thank you.
So, to sum up, we have discussed about the importance of carbon nitrogen compounds and how to prepare them specially for aromatic system, through the help of the nitro group and then by reduction and to convert the nitro to amine is very simple reduction and amine to diazo not much difficult, that is sodium nitrite (NaNO2), hydrochloric acid (HCl), diazotization and then by using Sandmeyer reaction, almost any functionality could be introduced where the diazo group will go, and the new group will come and the use of those compounds in the functional group transformation. I will continue with other aspects of the carbon-nitrogen compounds next time. Thank you.










