Chemistry Class 12 Unit 10 Chapter 04 Haloalkanes Haloarenes L 4 4 3P Jo4Xkdqa En Punc Para Txt
Hello everyone - I am Dr. Ramesh Rampanickar. I am an associate professor at the department of chemistry in Indian institute of technology Kanpur. So, in the last three lectures that I had given, I was talking about the chemistry of haloalkanes and haloarenes. So, today, I will continue to do that. So, this, as you already know, is from unit 10 of the NCERT textbook for chemistry, for class 12 students and what is left out of this unit to be discussed is the reactions of haloarenes. So, haloarenes, as you know, are compounds where halogen atom is attached to an aromatic compound. So, in the last class we had discussed the reactions of haloalkanes, and I also made it a point to mention that the reactivity pattern of haloalkanes is quite different from that of haloarenes. So, we would look at the reactions of haloarenes today.
In the reactions of haloalkanes one of the most interesting and most useful reactions was the nucleophilic substitution reaction. We said that if we have a halogen atom attached to an alkyl group, the halogen atom can be replaced by a number of different functional groups by using various nucleophiles. So, that was supposed to be - and that we have mentioned, is actually the most useful reaction and one of the best to make various derivatives of hydrocarbons. But now once we come to haloarenes quite interestingly, all the nucleophilic substitution reactions at first seem to be a possibility. These reactions do not work well, so, unlike alkyl halides, aryl halides are extremely slow and extremely sluggish when it comes to reaction with nucleophiles. So, there are various reasons for this, so we would look at the reasons one by one.
So, if you had a look at the screen here, you would find that one of the factors is a resonance effect. So, as you can see, whenever we have a halogen atom attached to an aromatic ring, the halogen atom that I have shown here is chlorobenzene , so, chlorine has lone pairs of electrons. So, these lone pairs of electrons, because the chlorine-carbon bond can rotate these lone pairs of electrons would come parallel to the electron cloud that is present in an aromatic ring. So, you know that an aromatic ring is stabilized by electron clouds on either side of it. So, similarly, when a chlorine atom comes, has this lone pair of electrons, they can come in parallel with the electron clouds present on an aromatic ring and start to interact with these electron clouds. So, they can have bonding interactions, which we normally call resonance structures that can be formed from this, or the effect is called the resonance effect.
So, this representation shows you how we would be able to draw them in simple chemical terms and show how a bonding interaction can happen between the lone pairs on chlorine and the aromatic ring. So, this is what we mean by resonance effect, so you can see that the lone pair of the chlorine atom is donated to the chlorine carbon bond effectively forming a double bonded compound. So, that means we have a chlorine carbon double bond, but now that chlorine has given its electrons to form this bond it receives a positive charge. But, however, when the double bond is formed, one of the double bonds in the ring within the aromatic compound also migrates to the adjacent carbon giving a negatively charged species. So, from a neutral structure, we have a structure that is charged with positive charge on chlorine, atom and negative charge on one of the carbon atoms. Now the negative charge does not stay on that particular carbon atom. It continues to move through the aromatic ring, so you would find that the negative charge then goes and forms a new double bond, while an existing double bond is now moved on to one of the carbon atoms to give you a new negatively charged carbon atom. Now this negatively charged then moves across the ring and forms a new double bond and negative charge is localized on another carbon atom.
So, all these structures are returned with the arrows that correspond to resonant structures. That means none of these structures really do exist. The actual structure is a mixture of all the structures that we have drawn here so out of the four structures we have. Three of them have a positively charged chlorine atom and all such compounds have a carbon chlorine double bond to so, therefore, this double bond character between carbon and chlorine makes it difficult to cleave the carbon-chloride bond, so, in effect, the carbon-chlorine bond has become shorter, it has a double bond and character, so it is much stronger than a single carbon-chlorine bond, and one interesting thing to note is that whenever we were writing haloalkanes, we always said that the carbon which is attached to the chlorine atom receives a slight positive charge, but whereas in the structures that we have now, you would find that chlorine is having a positive charge. It is because we have a double bond that is generated on chlorine at the expense of a lone pair of electrons that was present in chlorine. So, it has a double bond between carbon and chlorine, so a partial double bond between carbon and chlorine that results in lesser reactivity of this molecule. That means it is difficult to substitute that particular chlorine from the carbon atom now going ahead.
, so, chlorine has lone pairs of electrons. So, these lone pairs of electrons, because the chlorine-carbon bond can rotate these lone pairs of electrons would come parallel to the electron cloud that is present in an aromatic ring. So, you know that an aromatic ring is stabilized by electron clouds on either side of it. So, similarly, when a chlorine atom comes, has this lone pair of electrons, they can come in parallel with the electron clouds present on an aromatic ring and start to interact with these electron clouds. So, they can have bonding interactions, which we normally call resonance structures that can be formed from this, or the effect is called the resonance effect.
So, this representation shows you how we would be able to draw them in simple chemical terms and show how a bonding interaction can happen between the lone pairs on chlorine and the aromatic ring. So, this is what we mean by resonance effect, so you can see that the lone pair of the chlorine atom is donated to the chlorine carbon bond effectively forming a double bonded compound. So, that means we have a chlorine carbon double bond, but now that chlorine has given its electrons to form this bond it receives a positive charge. But, however, when the double bond is formed, one of the double bonds in the ring within the aromatic compound also migrates to the adjacent carbon giving a negatively charged species. So, from a neutral structure, we have a structure that is charged with positive charge on chlorine, atom and negative charge on one of the carbon atoms. Now the negative charge does not stay on that particular carbon atom. It continues to move through the aromatic ring, so you would find that the negative charge then goes and forms a new double bond, while an existing double bond is now moved on to one of the carbon atoms to give you a new negatively charged carbon atom. Now this negatively charged then moves across the ring and forms a new double bond and negative charge is localized on another carbon atom.
So, all these structures are returned with the arrows that correspond to resonant structures. That means none of these structures really do exist. The actual structure is a mixture of all the structures that we have drawn here so out of the four structures we have. Three of them have a positively charged chlorine atom and all such compounds have a carbon chlorine double bond to so, therefore, this double bond character between carbon and chlorine makes it difficult to cleave the carbon-chloride bond, so, in effect, the carbon-chlorine bond has become shorter, it has a double bond and character, so it is much stronger than a single carbon-chlorine bond, and one interesting thing to note is that whenever we were writing haloalkanes, we always said that the carbon which is attached to the chlorine atom receives a slight positive charge, but whereas in the structures that we have now, you would find that chlorine is having a positive charge. It is because we have a double bond that is generated on chlorine at the expense of a lone pair of electrons that was present in chlorine. So, it has a double bond between carbon and chlorine, so a partial double bond between carbon and chlorine that results in lesser reactivity of this molecule. That means it is difficult to substitute that particular chlorine from the carbon atom now going ahead.
 A second reason for this is the difference in hybridization of carbon atom in C-X bond. So, I have two structures here. One of them is an aryl halide, a haloarene and another one is an alkyl halide. So, if you look at the carbon that has bonded to the halogen atom in a haloarene, it is a sp2 hybridized carbon atom. So, what do we exactly mean by a sp2 hybridized carbon atom? Is that the amount of s character on the orbital that is being used by carbon or the atomic orbital that is used by carbon is more so whenever we increase the s character in an orbital. The orbital becomes more electronegative, because s is the inner shell. So, therefore, you would find that the increased percentage of s or s character in the orbital makes this particular orbital more electronegative, or in other words the carbon that is bonded to X in an in a haloarene, is more electronegative than the carbon that is bonded to a halogen atom in a haloalkane. So, therefore, because the carbon is now more electronegative, it does not allow the bond to be polarized as much as it is polarized in a haloalkane or in other words the electron that you find between carbon and halogen is not moved too much towards chlorine. So, the extent of polarization is less. The bond therefore happens to be shorter.
So, if you compare chloroalkene and chloroarene generally, it is found that the chloroarene has a shorter carbon-chlorine bond in comparison with chloroalkane. So, this shorter bond also means that it is stronger, and this also can be attributed to the fact that the carbon-chloride bond has a double bond character, which we saw before. So, because of these factors, it becomes difficult to cleave this bond. So, that is the whole point. So, if you want to do a substitution with the nucleophile, we would like to break the carbon chlorine bond so that becomes difficult.
Now then, we can also think about the other mechanism that was ah possible for haloalkanes, which was SN1 substitution reactions, where we have to assume that the halogen leaves from the molecule and gives the positive charge to the carbon atom to which it is attached. This is difficult because the bond is not polarized and even if we assume that, under forcing conditions, we were to force an SN1 reaction on a haloarene, you will find that the positive charge now has to rest on an orbital which is sp2 hybridized. So, carbon uses a sp2 hybridized orbital to form the carbon-chlorine bond. Now when the chlorine leaves with its electrons, the sp2 orbital is now empty or it or therefore, the carbon receives a positive charge and that orbital.
So, the problem with having that positive charge is all the aromatic ring is electron rich. If this, if my palm is to be suppose, is supposed as the aromatic ring, you would find that the electron clouds are on either side of this. So, you have electron clouds on top and bottom of the aromatic ring. Now the orbital that has the positive charge also lies along the plane of this, so it is in one plane. So, whenever we generate a positive charge, it is generated on the aromatic ring. That particular orbital is in the plane of the aromatic ring and therefore that empty orbital cannot be supported by the electron clouds on either side, because this is actually in the node between the two components of the electron cloud that is available on aromatic ring, so this cannot be resonant stabilized, so that is the trouble we have so therefore, this aryl cation is extremely unstable, so there are two reasons:
One of them the empty orbital is known as sp2 orbital, which is more electronegative, so the carbon starts to feel more positive charge, and the second reason is that this particular positive charge or the absence of electrons, cannot be supported by the electron cloud that is present in the aromatic ring, because it kind of falls in the nodes of this electron cloud, so that makes an SN1 mechanism practically impossible for haloarenes. So, we have seen that the SN2 reaction required that we cleave the carbon-halogen bond when the nucleophile approaches and the SN1 reaction requires that it cleaves even before, so both these are not possible, and there is also another reason why SN2 reactions are not possible is because aromatic rings are electron rich, they have an aromatic electron cloud, a nucleophile is also electron rich. So, when two electron rich species have to come together for a reaction to happen, you normally find that there is a large amount of repulsion between the electron rich species and that results in the reaction being slower. So, these factors, these four factors that it is. I have discussed now there is resonance, the difference in hybridization, instability of aryl cations and finally, the repulsion between a nucleophile and an aromatic ring. So, all these factors contribute together and make nucleophilic substitution reactions of aromatic compounds extremely difficult.
A second reason for this is the difference in hybridization of carbon atom in C-X bond. So, I have two structures here. One of them is an aryl halide, a haloarene and another one is an alkyl halide. So, if you look at the carbon that has bonded to the halogen atom in a haloarene, it is a sp2 hybridized carbon atom. So, what do we exactly mean by a sp2 hybridized carbon atom? Is that the amount of s character on the orbital that is being used by carbon or the atomic orbital that is used by carbon is more so whenever we increase the s character in an orbital. The orbital becomes more electronegative, because s is the inner shell. So, therefore, you would find that the increased percentage of s or s character in the orbital makes this particular orbital more electronegative, or in other words the carbon that is bonded to X in an in a haloarene, is more electronegative than the carbon that is bonded to a halogen atom in a haloalkane. So, therefore, because the carbon is now more electronegative, it does not allow the bond to be polarized as much as it is polarized in a haloalkane or in other words the electron that you find between carbon and halogen is not moved too much towards chlorine. So, the extent of polarization is less. The bond therefore happens to be shorter.
So, if you compare chloroalkene and chloroarene generally, it is found that the chloroarene has a shorter carbon-chlorine bond in comparison with chloroalkane. So, this shorter bond also means that it is stronger, and this also can be attributed to the fact that the carbon-chloride bond has a double bond character, which we saw before. So, because of these factors, it becomes difficult to cleave this bond. So, that is the whole point. So, if you want to do a substitution with the nucleophile, we would like to break the carbon chlorine bond so that becomes difficult.
Now then, we can also think about the other mechanism that was ah possible for haloalkanes, which was SN1 substitution reactions, where we have to assume that the halogen leaves from the molecule and gives the positive charge to the carbon atom to which it is attached. This is difficult because the bond is not polarized and even if we assume that, under forcing conditions, we were to force an SN1 reaction on a haloarene, you will find that the positive charge now has to rest on an orbital which is sp2 hybridized. So, carbon uses a sp2 hybridized orbital to form the carbon-chlorine bond. Now when the chlorine leaves with its electrons, the sp2 orbital is now empty or it or therefore, the carbon receives a positive charge and that orbital.
So, the problem with having that positive charge is all the aromatic ring is electron rich. If this, if my palm is to be suppose, is supposed as the aromatic ring, you would find that the electron clouds are on either side of this. So, you have electron clouds on top and bottom of the aromatic ring. Now the orbital that has the positive charge also lies along the plane of this, so it is in one plane. So, whenever we generate a positive charge, it is generated on the aromatic ring. That particular orbital is in the plane of the aromatic ring and therefore that empty orbital cannot be supported by the electron clouds on either side, because this is actually in the node between the two components of the electron cloud that is available on aromatic ring, so this cannot be resonant stabilized, so that is the trouble we have so therefore, this aryl cation is extremely unstable, so there are two reasons:
One of them the empty orbital is known as sp2 orbital, which is more electronegative, so the carbon starts to feel more positive charge, and the second reason is that this particular positive charge or the absence of electrons, cannot be supported by the electron cloud that is present in the aromatic ring, because it kind of falls in the nodes of this electron cloud, so that makes an SN1 mechanism practically impossible for haloarenes. So, we have seen that the SN2 reaction required that we cleave the carbon-halogen bond when the nucleophile approaches and the SN1 reaction requires that it cleaves even before, so both these are not possible, and there is also another reason why SN2 reactions are not possible is because aromatic rings are electron rich, they have an aromatic electron cloud, a nucleophile is also electron rich. So, when two electron rich species have to come together for a reaction to happen, you normally find that there is a large amount of repulsion between the electron rich species and that results in the reaction being slower. So, these factors, these four factors that it is. I have discussed now there is resonance, the difference in hybridization, instability of aryl cations and finally, the repulsion between a nucleophile and an aromatic ring. So, all these factors contribute together and make nucleophilic substitution reactions of aromatic compounds extremely difficult.
 It does not mean that we cannot carry out these reactions. We can, of course, do these reactions, but some of these reactions would require extremely hard conditions, in comparison with what was required for the reactions of haloalkanes. So, I have an example here, so these are reactions of chloroarenes with hydroxide ions (OH-), so hydroxide ions are nucleophile. So, let us take the first example that is given here. So, if you take chlorobenzene
It does not mean that we cannot carry out these reactions. We can, of course, do these reactions, but some of these reactions would require extremely hard conditions, in comparison with what was required for the reactions of haloalkanes. So, I have an example here, so these are reactions of chloroarenes with hydroxide ions (OH-), so hydroxide ions are nucleophile. So, let us take the first example that is given here. So, if you take chlorobenzene and treat it with sodium hydroxide (NaOH), then the condition that is required is 623 kelvins, so it is approximately 300 and it is 350 degrees Celsius and 300 atmospheres. So, the reaction requires extremely high pressure and very high temperature. Only then does the nucleophilic substitution happen. So, the nucleophilic substitution of chlorine with the hydroxide anion (OH-) is possible, provided we will give extremely harsh conditions, including very high temperature and very high pressure. Now you can see that there are two steps to the reaction. One is where you treat it with sodium hydroxide (NaOH) at the temperature and pressure that you mentioned and in the second step the same molecule is treated with an acid, so H+ here is required because under basic condition, the phenol
and treat it with sodium hydroxide (NaOH), then the condition that is required is 623 kelvins, so it is approximately 300 and it is 350 degrees Celsius and 300 atmospheres. So, the reaction requires extremely high pressure and very high temperature. Only then does the nucleophilic substitution happen. So, the nucleophilic substitution of chlorine with the hydroxide anion (OH-) is possible, provided we will give extremely harsh conditions, including very high temperature and very high pressure. Now you can see that there are two steps to the reaction. One is where you treat it with sodium hydroxide (NaOH) at the temperature and pressure that you mentioned and in the second step the same molecule is treated with an acid, so H+ here is required because under basic condition, the phenol that is formed would be a phenoxide anion because phenol
that is formed would be a phenoxide anion because phenol is acidic so in the presence of sodium hydroxide (NaOH) after the first step, the product you get will be a sodium salt of phenol
is acidic so in the presence of sodium hydroxide (NaOH) after the first step, the product you get will be a sodium salt of phenol , so, you have to neutralize it. That is why we have H+ as a second step, okay?
Now in the second reaction, what you are seeing is this: we have the same substrate, but we have added a NO2 on para position to the chlorine. So, when we add a NO2 the para position that this is a mono substituted chlorobenzene or a chloronitrobenzene
, so, you have to neutralize it. That is why we have H+ as a second step, okay?
Now in the second reaction, what you are seeing is this: we have the same substrate, but we have added a NO2 on para position to the chlorine. So, when we add a NO2 the para position that this is a mono substituted chlorobenzene or a chloronitrobenzene . So, in this particular case we have this nitro group present and then there is a dramatic difference in the conditions that are required earlier. We needed a much higher temperature and very high pressure here. The pressure factor has been removed, so this reaction happens at atmospheric pressure and at not so high temperature, so 443 kelvins approximately 175 degrees Celsius. So, the reaction would happen at a slightly lower temperature than what was earlier required, and it gives us the product after a protonation using an acid. Ok.
Now, in the third example, we have added one more nitro group and we see that the trend continues. So, as we keep on increasing the number of nitro groups in aromatic ring, the reaction conditions become milder and milder. So, under this condition you need less than 100 degrees Celsius for the reaction to be carried out and there is no requirement of higher pressure, so we get the product, which is dinitrophenol
. So, in this particular case we have this nitro group present and then there is a dramatic difference in the conditions that are required earlier. We needed a much higher temperature and very high pressure here. The pressure factor has been removed, so this reaction happens at atmospheric pressure and at not so high temperature, so 443 kelvins approximately 175 degrees Celsius. So, the reaction would happen at a slightly lower temperature than what was earlier required, and it gives us the product after a protonation using an acid. Ok.
Now, in the third example, we have added one more nitro group and we see that the trend continues. So, as we keep on increasing the number of nitro groups in aromatic ring, the reaction conditions become milder and milder. So, under this condition you need less than 100 degrees Celsius for the reaction to be carried out and there is no requirement of higher pressure, so we get the product, which is dinitrophenol  as the product. In this case trinitro chlorobenzene
as the product. In this case trinitro chlorobenzene . So, here there are three nitro groups on the two ortho position and the para position. So, if you would like to name it other ways, we can say that this is 2, 4 and 6 positions of the chlorobenzene
. So, here there are three nitro groups on the two ortho position and the para position. So, if you would like to name it other ways, we can say that this is 2, 4 and 6 positions of the chlorobenzene . We have nitro substitutions, but now you would find that the condition is extremely simple, and this reaction works almost like the reactions of alkyl halides. Here all you need to do is take water and warm the reaction mixture. So, we do not even need sodium hydroxide (NaOH), where an OH- nucleophile has to react. Instead, water, with its lone pairs, will be able to do a nucleophilic substitution reaction on this substrate and give us this product, which is picric acid
. We have nitro substitutions, but now you would find that the condition is extremely simple, and this reaction works almost like the reactions of alkyl halides. Here all you need to do is take water and warm the reaction mixture. So, we do not even need sodium hydroxide (NaOH), where an OH- nucleophile has to react. Instead, water, with its lone pairs, will be able to do a nucleophilic substitution reaction on this substrate and give us this product, which is picric acid . This particular product is called picric acid. Now what we have seen is that a chloroarenes are sluggish. They do not give you nucleophilic substitution reactions. You have to really force the conditions, but as we keep on adding a group such as nitro, so nitro specifically here, because nitro is an electron withdrawing group. So, what we are actually doing to the substrates here by increasing the number of nitro groups is to make the aromatic ring electron deficient. So, an aromatic ring was electron rich earlier. So, once you put a nitro group, the nitro group pulls the electron towards itself. So, therefore, the aromatic ring slowly starts to become electron deficient and that would make the approach of the nucleophile to the aromatic ring easier and also the aromatic ring would be able to handle a negative charge. So, we would look at the mechanism soon and then we will find out how this reaction works.
. This particular product is called picric acid. Now what we have seen is that a chloroarenes are sluggish. They do not give you nucleophilic substitution reactions. You have to really force the conditions, but as we keep on adding a group such as nitro, so nitro specifically here, because nitro is an electron withdrawing group. So, what we are actually doing to the substrates here by increasing the number of nitro groups is to make the aromatic ring electron deficient. So, an aromatic ring was electron rich earlier. So, once you put a nitro group, the nitro group pulls the electron towards itself. So, therefore, the aromatic ring slowly starts to become electron deficient and that would make the approach of the nucleophile to the aromatic ring easier and also the aromatic ring would be able to handle a negative charge. So, we would look at the mechanism soon and then we will find out how this reaction works.
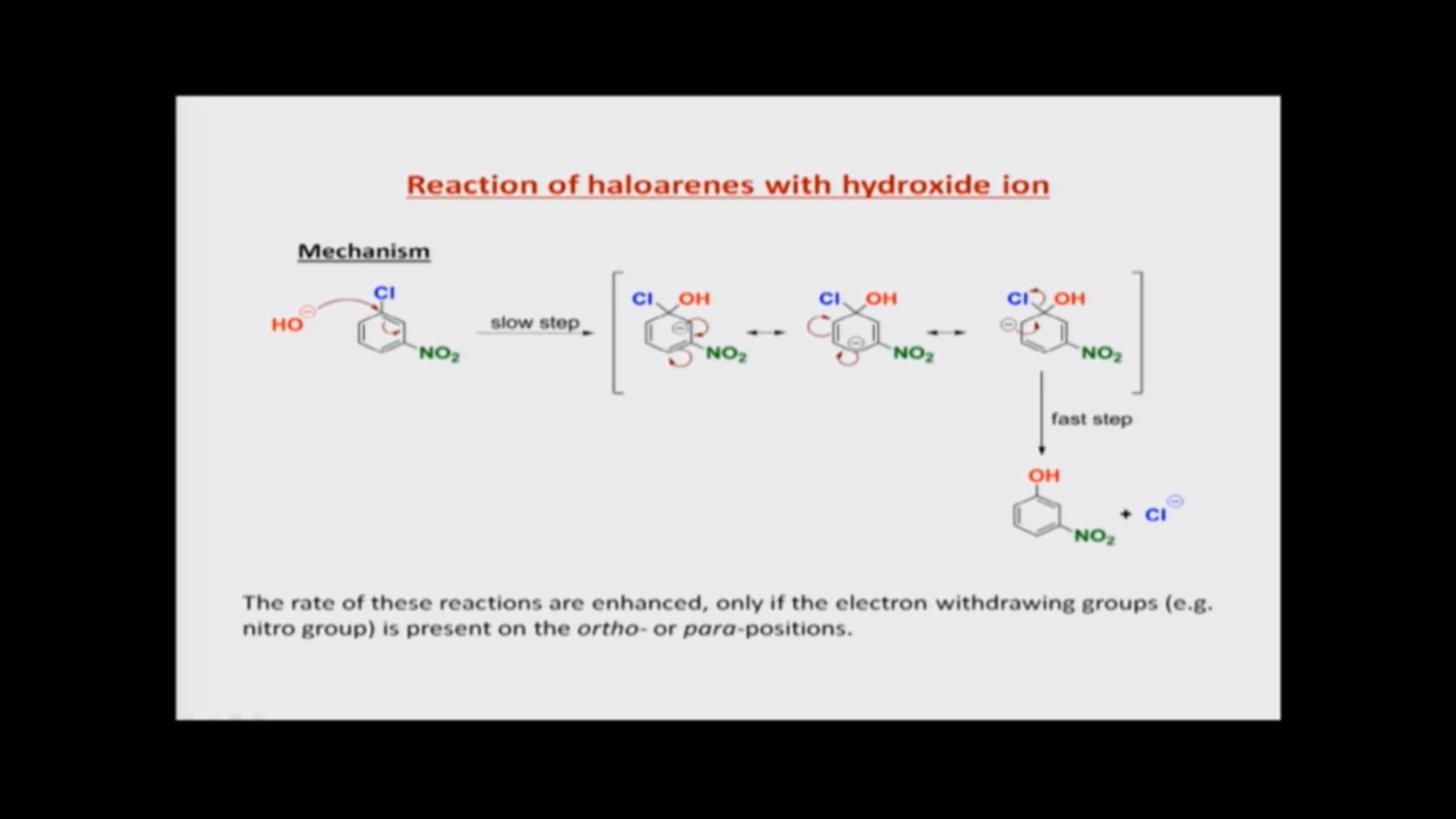
 So, on this page you can see the mechanism of this particular reaction. So, what I have first here is the para-nitro chlorobenzene so or para-chloro nitrobenzene
So, on this page you can see the mechanism of this particular reaction. So, what I have first here is the para-nitro chlorobenzene so or para-chloro nitrobenzene it has to be called. So, you would find that in the para-substituted compound the reaction follows a very interesting path. It is not like SN1 or SN2 reactions the OH-, starts to attack on the carbon atom that is attached to chlorine, and then we get an intermediate where there is a carbon atom that is attached both to chlorine and to the hydroxide group. Now the double bond that was present with that carbon atom has moved on to the adjacent carbon with the forming a negatively charged carbon there. So, there is a carbanion formed and, and we have a carbon that is tetrahedral that is attached to four different groups. Ok and now what happens? The negative charge is then delocalized throughout the ring similar to the resonance structures that we drew earlier. So, the negative charge moves on and then a new double bond is formed and then now we have the negative. The carbanion on the carbon that is attached to nitro group and then it moves further, and the carbanion reaches here. And finally, when the double bond is reinstated, the chlorine atom can come out as a chloride ion.
So effectively, if you look at the structure on extreme left and the structure on extreme structures on extreme right, you see that this is a nucleophilic substitution reaction. The chlorine atom has been replaced by the hydroxide ion (OH-). But, however, there are these intermediates, and these intermediates are called Meisenheimer complexes, so these intermediates that are written here are called Meisenheimer complexes. So, these are compounds where we have a nucleophile added onto an aromatic ring by giving a negative charge on the aromatic ring. Now, our aromatic ring in this case is substituted. We have substituted it with a nitro group, so you also see the nitro group is electron withdrawing and in at least one of the structures, one of the three structures that I have written in square brackets here you can see that the negative is on the carbon atom that is attached to the nitro group. So, that means this negative charge also can be delocalized on to the nitro group. So, therefore, the nitro group, which is electron withdrawing, will be able to pull the electrons towards itself and stabilize the Meisenheimer complex, so Meisenheimer complex is burned. A nucleophile adds on to haloarene and forms a negatively charged species along with the tetrahedral carbon atom, and now such species are stabilized whenever there are electron withdrawing groups present on the carbon atoms, where the negative charge starts to appear.
So, there are three structures that represent the Meisenheimer complex and in one of them the negative charge is on the carbon atom that is attached to nitro group. So, that makes this reaction happen now the first step of the reaction where the OH- has to come and start forming the tetrahedral carbon atom. This is the slower step in the reaction. It is reasonable because now we are talking about breaking the aromaticity of the aromatic ring, so here we had an aromatic ring very much intact. Now, once we start forming this tetrahedral carbon atom here, the aromaticity of the molecule is lost. So, this is there for a very slow process, but after once this happened. The elimination of chloride as an anion is extremely fast. So, the last step that means the breakdown of the Meisenheimer complex into the products is faster. So, the first step is slower, whereas the second step is faster. So, that is how this reaction works.
Now I have the same mechanism for an ortho nitro derivative, so that means I have ortho-chloride nitrobenzene
it has to be called. So, you would find that in the para-substituted compound the reaction follows a very interesting path. It is not like SN1 or SN2 reactions the OH-, starts to attack on the carbon atom that is attached to chlorine, and then we get an intermediate where there is a carbon atom that is attached both to chlorine and to the hydroxide group. Now the double bond that was present with that carbon atom has moved on to the adjacent carbon with the forming a negatively charged carbon there. So, there is a carbanion formed and, and we have a carbon that is tetrahedral that is attached to four different groups. Ok and now what happens? The negative charge is then delocalized throughout the ring similar to the resonance structures that we drew earlier. So, the negative charge moves on and then a new double bond is formed and then now we have the negative. The carbanion on the carbon that is attached to nitro group and then it moves further, and the carbanion reaches here. And finally, when the double bond is reinstated, the chlorine atom can come out as a chloride ion.
So effectively, if you look at the structure on extreme left and the structure on extreme structures on extreme right, you see that this is a nucleophilic substitution reaction. The chlorine atom has been replaced by the hydroxide ion (OH-). But, however, there are these intermediates, and these intermediates are called Meisenheimer complexes, so these intermediates that are written here are called Meisenheimer complexes. So, these are compounds where we have a nucleophile added onto an aromatic ring by giving a negative charge on the aromatic ring. Now, our aromatic ring in this case is substituted. We have substituted it with a nitro group, so you also see the nitro group is electron withdrawing and in at least one of the structures, one of the three structures that I have written in square brackets here you can see that the negative is on the carbon atom that is attached to the nitro group. So, that means this negative charge also can be delocalized on to the nitro group. So, therefore, the nitro group, which is electron withdrawing, will be able to pull the electrons towards itself and stabilize the Meisenheimer complex, so Meisenheimer complex is burned. A nucleophile adds on to haloarene and forms a negatively charged species along with the tetrahedral carbon atom, and now such species are stabilized whenever there are electron withdrawing groups present on the carbon atoms, where the negative charge starts to appear.
So, there are three structures that represent the Meisenheimer complex and in one of them the negative charge is on the carbon atom that is attached to nitro group. So, that makes this reaction happen now the first step of the reaction where the OH- has to come and start forming the tetrahedral carbon atom. This is the slower step in the reaction. It is reasonable because now we are talking about breaking the aromaticity of the aromatic ring, so here we had an aromatic ring very much intact. Now, once we start forming this tetrahedral carbon atom here, the aromaticity of the molecule is lost. So, this is there for a very slow process, but after once this happened. The elimination of chloride as an anion is extremely fast. So, the last step that means the breakdown of the Meisenheimer complex into the products is faster. So, the first step is slower, whereas the second step is faster. So, that is how this reaction works.
Now I have the same mechanism for an ortho nitro derivative, so that means I have ortho-chloride nitrobenzene . So, now, in this particular structure, you would particularly compound you find that it is. You will find that it is the exact same thing that is happening: the OH- attacks. It forms a tetrahedral carbon atom. The negative charge is now already on the carbon atom that is attached to nitro, so very good, so the negative charge can be delocalized onto the nitro group and stabilized. Now the resonance structures continue to form. That means the negative charge continues to move through the aromatic ring, and we can again draw three Meisenheimer complex structures, all of them in resonance so effectively. The negative charge is sent through the five carbon atoms that are present in this complex, and only one of the carbon atoms is tetrahedron structure. So, one way of drawing Meisenheimer complexes that you would find being used by people is to draw the structure like this with the negative charge and then a chloride, OH and a nitro at whichever position you would like to put it either ortho or para. So, there is this negative charge that is delocalized through this part of the molecule, and we have tetrahedral carbon there. So, this is how normally a Meisenheimer complex is represented with a single structure. Otherwise, we will have to draw all three structures to represent it correctly now here again, the first step is elimination of this.
So, in these two structures, these two examples that I have drawn. I have the nitro group on ortho and para positions and in both the Meisenheimer complexes those are formed. You find that the negative charge is on a carbon atom that is near to the nitro that is attached to the nitro group. So, in the second case, it is on carbon number two and in the first case it is on carbon number four. So, these structures are something that we need to remember, because those are the ones which make this reaction faster.
. So, now, in this particular structure, you would particularly compound you find that it is. You will find that it is the exact same thing that is happening: the OH- attacks. It forms a tetrahedral carbon atom. The negative charge is now already on the carbon atom that is attached to nitro, so very good, so the negative charge can be delocalized onto the nitro group and stabilized. Now the resonance structures continue to form. That means the negative charge continues to move through the aromatic ring, and we can again draw three Meisenheimer complex structures, all of them in resonance so effectively. The negative charge is sent through the five carbon atoms that are present in this complex, and only one of the carbon atoms is tetrahedron structure. So, one way of drawing Meisenheimer complexes that you would find being used by people is to draw the structure like this with the negative charge and then a chloride, OH and a nitro at whichever position you would like to put it either ortho or para. So, there is this negative charge that is delocalized through this part of the molecule, and we have tetrahedral carbon there. So, this is how normally a Meisenheimer complex is represented with a single structure. Otherwise, we will have to draw all three structures to represent it correctly now here again, the first step is elimination of this.
So, in these two structures, these two examples that I have drawn. I have the nitro group on ortho and para positions and in both the Meisenheimer complexes those are formed. You find that the negative charge is on a carbon atom that is near to the nitro that is attached to the nitro group. So, in the second case, it is on carbon number two and in the first case it is on carbon number four. So, these structures are something that we need to remember, because those are the ones which make this reaction faster.
 Now I would go on and see what happens when the nitro group is in meta position. So, that means when we have meta-chloronitrobenzene
Now I would go on and see what happens when the nitro group is in meta position. So, that means when we have meta-chloronitrobenzene here again, the OH- can come and attack here. So, when an OH- attacks we generate a negative charge just like in the other cases, we do have the Meisenheimer complex and now the negative charge forms a new double bond and the negative charge move through the ring by forming a new double bond and moving the pair of electrons through the aromatic ring. We get the second structure and finally, the third now in this sequence of reaction in the in this series of Meisenheimer complex structures that we have drawn, you would find that the negative charge is never on the carbon atom that is attached to the nitro group. So, in this it is not on the carbon that is attached to nitro group. Here again, it is not the case and not even in this. So, therefore, although nitro group is something that can withdraw electrons, it can stabilize the negative charge better if the negative charge comes on the carbon atom to which the nitro group is attached. Now, if none of the structures, the negative charge comes to the carbon atom to which nitro group is attached, then the negative charge is not stabilized. So, therefore, a substitution of an electron withdrawing group such as the nitro group on meta position, does not make this reaction faster.
Once again, we saw that chlorobenzene reacts very slowly to nucleophilic substitution reactions. We saw that, if the number of nitro groups increases on ortho and para positions, the rate of the reaction increases the condition required to carry out the reaction becomes milder and milder. However, if the nitro group is present in a meta position, this does not happen. So, in short, we would be able to say that the rate of these reactions is enhanced only if the electron withdrawing group, such as the nitro group, is present on ortho and para positions, and if they are present on meta positions, then the reactions do not happen faster, ok.
here again, the OH- can come and attack here. So, when an OH- attacks we generate a negative charge just like in the other cases, we do have the Meisenheimer complex and now the negative charge forms a new double bond and the negative charge move through the ring by forming a new double bond and moving the pair of electrons through the aromatic ring. We get the second structure and finally, the third now in this sequence of reaction in the in this series of Meisenheimer complex structures that we have drawn, you would find that the negative charge is never on the carbon atom that is attached to the nitro group. So, in this it is not on the carbon that is attached to nitro group. Here again, it is not the case and not even in this. So, therefore, although nitro group is something that can withdraw electrons, it can stabilize the negative charge better if the negative charge comes on the carbon atom to which the nitro group is attached. Now, if none of the structures, the negative charge comes to the carbon atom to which nitro group is attached, then the negative charge is not stabilized. So, therefore, a substitution of an electron withdrawing group such as the nitro group on meta position, does not make this reaction faster.
Once again, we saw that chlorobenzene reacts very slowly to nucleophilic substitution reactions. We saw that, if the number of nitro groups increases on ortho and para positions, the rate of the reaction increases the condition required to carry out the reaction becomes milder and milder. However, if the nitro group is present in a meta position, this does not happen. So, in short, we would be able to say that the rate of these reactions is enhanced only if the electron withdrawing group, such as the nitro group, is present on ortho and para positions, and if they are present on meta positions, then the reactions do not happen faster, ok.
 So, that is about nucleophilic substitution reactions of haloarenes. So, you would already realize that it is a tough reaction to do so. People do not normally go for that, but however aromatic rings because of its electron cloud because of its rich electronic species, those are present the aromatic ring itself. They tend to give you another reaction which alkyl halides cannot give and those are electrophilic substitution reactions. You have already learned them probably while you are learning aromatic compounds, so electrophilic substitution reactions do happen in haloarenes. Now, what does a halogen atom do to an aromatic ring?
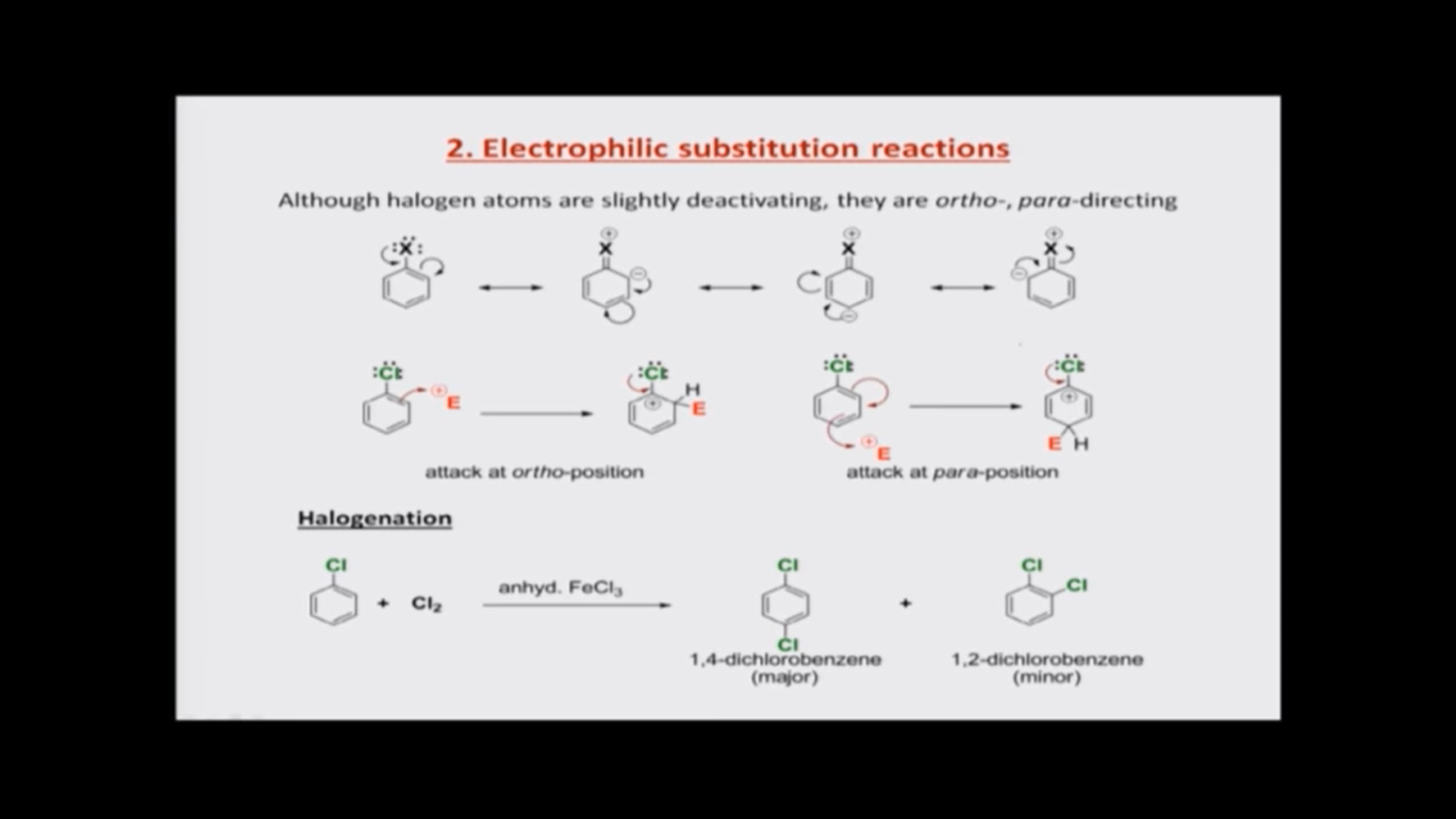
So, we are now going to discuss haloarenes. That means these are aromatic rings that are attached to a halogen atom. So, let us see, what can a halogen do to an aromatic ring, so halogen atom by itself is something that will pull electrons out because the carbon-chlorine bond, the carbon-halogen bond, the halogen pulls electrons. So, they are slightly deactivating, so they tend to deactivate the aromatic ring by deactivation of an aromatic ring. We mean that an aromatic ring loses its electron density to a substituent, so the substituent is something that pulls electrons slightly from the aromatic ring and makes the aromatic ring feel less electron rich, so halogens do that. But however, halogen atoms also have this lone pair. So, these are structures we already drew once so. These lone pairs of electrons in halogen atoms can be delocalized onto the rings to get this structure, so it can go with the negative charge on ortho position, para position and on the other ortho position and in such structures, we have this carbon halogen double bond. So, this is something we saw, and the halogen gets a positive charge too.
So, there are two things now: one of them is the halogen atom pulls electrons from the aromatic ring, because it is an electron negative atom. So, therefore, the aromatic ring is now electron deficient at the same time, although it makes the aromatic bring electron deficient, whatever electron density is available on the aromatic ring. It is enhanced on ortho and para positions, because in these resonance structures you can see that there are negative charges which are in structure one and three you will find that the negative charges are on ortho position to the halogen atom and in structure two, which I am showing you now. The negative charge is on the carbon atom 4. So, therefore, these structures are favor substitutions on these positions, so an electrophile. So, once again, electrophiles are those species which have a positive charge or those which are electron deficient and are looking for an electron rich species to react with. So, when an electrophile approaches a haloarene, it sees that the haloarene is not so easily reacting. But, however, if it has to react, it will try to react through ortho and para positions of the halogen atom. It is because those are the ones that have negative charges in the resonance structures.
So, here I have these structures representing an ortho attack and an and a para-attack that means attack at an ortho position and at a para position, so simply drawing we would be able to draw that the double bond present in the aromatic ring would migrate to react with the electrophile which is shown as E having a positive charge. Then a new bond is formed. Of course, this carbon atom has a hydrogen atom, so we say that this carbon is now a tetrahedral and the positive charge resides on the carbon atom that has chlorine. Now, the presence of a positive charge adjacent to chlorine atom is not good because chlorine is electronegative. So, it does not want the positive charge there. So, that is why we say the ring is deactivated. But, however, once the positive charge comes there, the lone pairs can stabilize the positive charge, so that also helps in the ortho attack. So, if the attack has to happen, it can happen in ortho. Similarly, if the attack has to happen in para position, we have a new carbon-E bond where E is the electrophile, and that carbon now is tetrahedral and if you draw two arrows, as I have shown here, you will be able to see that the positive charge is now on the carbon that is attached to chlorine and chlorine will be able to stabilize the positive charge using its lone pair. So, these are the reasons why ortho and para positions, substitutions at ortho and para positions can be stabilized by halogen, whereas if the substitution was at meta position, the positive charge will not come on to the carbon having chlorine and therefore a resonant stabilization will not be possible so you may you can draw those structures yourself and feel for it. Okay.
So, now let us look at some of the most useful electrophilic substitution reactions of haloarenes, so the first reaction is halogenation itself. So, that means, if we have a haloarenes, we can add more halogen atoms to that. So, this is a reaction we have learnt while we are talking about the preparative methods for haloarenes, so you can take a haloarene treat it with another halogen with halogen molecule, either chlorine or bromine in the presence of anhydrous FeCl3 or Fe itself, so which would form a FeCl3 which acts as a lewis acid and FeCl3 would then react with chlorine forming what will be FeCl4- + Cl+, so the Cl+ that is formed would be the electrophile. So, if you look at the reactions of that electrophile that I mentioned earlier, where the electrophile is shown, as shown in red color here, that electrophile is Cl+. Now Cl, the electrophile thus formed would then react on ortho and para positions as expected, and normally you would also find that the substitution on para position is more favored. It is just because two substitutions on ortho position. That means 1, 2 substitutions on aromatic ring, as in this case we can see here 1, 2-dichlorobenzene
So, that is about nucleophilic substitution reactions of haloarenes. So, you would already realize that it is a tough reaction to do so. People do not normally go for that, but however aromatic rings because of its electron cloud because of its rich electronic species, those are present the aromatic ring itself. They tend to give you another reaction which alkyl halides cannot give and those are electrophilic substitution reactions. You have already learned them probably while you are learning aromatic compounds, so electrophilic substitution reactions do happen in haloarenes. Now, what does a halogen atom do to an aromatic ring?
So, we are now going to discuss haloarenes. That means these are aromatic rings that are attached to a halogen atom. So, let us see, what can a halogen do to an aromatic ring, so halogen atom by itself is something that will pull electrons out because the carbon-chlorine bond, the carbon-halogen bond, the halogen pulls electrons. So, they are slightly deactivating, so they tend to deactivate the aromatic ring by deactivation of an aromatic ring. We mean that an aromatic ring loses its electron density to a substituent, so the substituent is something that pulls electrons slightly from the aromatic ring and makes the aromatic ring feel less electron rich, so halogens do that. But however, halogen atoms also have this lone pair. So, these are structures we already drew once so. These lone pairs of electrons in halogen atoms can be delocalized onto the rings to get this structure, so it can go with the negative charge on ortho position, para position and on the other ortho position and in such structures, we have this carbon halogen double bond. So, this is something we saw, and the halogen gets a positive charge too.
So, there are two things now: one of them is the halogen atom pulls electrons from the aromatic ring, because it is an electron negative atom. So, therefore, the aromatic ring is now electron deficient at the same time, although it makes the aromatic bring electron deficient, whatever electron density is available on the aromatic ring. It is enhanced on ortho and para positions, because in these resonance structures you can see that there are negative charges which are in structure one and three you will find that the negative charges are on ortho position to the halogen atom and in structure two, which I am showing you now. The negative charge is on the carbon atom 4. So, therefore, these structures are favor substitutions on these positions, so an electrophile. So, once again, electrophiles are those species which have a positive charge or those which are electron deficient and are looking for an electron rich species to react with. So, when an electrophile approaches a haloarene, it sees that the haloarene is not so easily reacting. But, however, if it has to react, it will try to react through ortho and para positions of the halogen atom. It is because those are the ones that have negative charges in the resonance structures.
So, here I have these structures representing an ortho attack and an and a para-attack that means attack at an ortho position and at a para position, so simply drawing we would be able to draw that the double bond present in the aromatic ring would migrate to react with the electrophile which is shown as E having a positive charge. Then a new bond is formed. Of course, this carbon atom has a hydrogen atom, so we say that this carbon is now a tetrahedral and the positive charge resides on the carbon atom that has chlorine. Now, the presence of a positive charge adjacent to chlorine atom is not good because chlorine is electronegative. So, it does not want the positive charge there. So, that is why we say the ring is deactivated. But, however, once the positive charge comes there, the lone pairs can stabilize the positive charge, so that also helps in the ortho attack. So, if the attack has to happen, it can happen in ortho. Similarly, if the attack has to happen in para position, we have a new carbon-E bond where E is the electrophile, and that carbon now is tetrahedral and if you draw two arrows, as I have shown here, you will be able to see that the positive charge is now on the carbon that is attached to chlorine and chlorine will be able to stabilize the positive charge using its lone pair. So, these are the reasons why ortho and para positions, substitutions at ortho and para positions can be stabilized by halogen, whereas if the substitution was at meta position, the positive charge will not come on to the carbon having chlorine and therefore a resonant stabilization will not be possible so you may you can draw those structures yourself and feel for it. Okay.
So, now let us look at some of the most useful electrophilic substitution reactions of haloarenes, so the first reaction is halogenation itself. So, that means, if we have a haloarenes, we can add more halogen atoms to that. So, this is a reaction we have learnt while we are talking about the preparative methods for haloarenes, so you can take a haloarene treat it with another halogen with halogen molecule, either chlorine or bromine in the presence of anhydrous FeCl3 or Fe itself, so which would form a FeCl3 which acts as a lewis acid and FeCl3 would then react with chlorine forming what will be FeCl4- + Cl+, so the Cl+ that is formed would be the electrophile. So, if you look at the reactions of that electrophile that I mentioned earlier, where the electrophile is shown, as shown in red color here, that electrophile is Cl+. Now Cl, the electrophile thus formed would then react on ortho and para positions as expected, and normally you would also find that the substitution on para position is more favored. It is just because two substitutions on ortho position. That means 1, 2 substitutions on aromatic ring, as in this case we can see here 1, 2-dichlorobenzene . So, when you have substitutions on adjacent carbon atoms on an aromatic ring, there would be some steric hindrance, because if you look at this double bond, this double bond is now as if it is a cis double bond with both the chlorine atoms are on same side. So, it is like a double bond having cis substitution, so they are very close, so there would be some sort of repulsion between them, so normally you would find that the para substitutions are favored in electrophilic substitution reaction.
So, halogenation gives you two products, a mixture of ortho and para substituted, compound 1, 4-dichlorobenzene
. So, when you have substitutions on adjacent carbon atoms on an aromatic ring, there would be some steric hindrance, because if you look at this double bond, this double bond is now as if it is a cis double bond with both the chlorine atoms are on same side. So, it is like a double bond having cis substitution, so they are very close, so there would be some sort of repulsion between them, so normally you would find that the para substitutions are favored in electrophilic substitution reaction.
So, halogenation gives you two products, a mixture of ortho and para substituted, compound 1, 4-dichlorobenzene and 1, 2-dichlorobenzene
and 1, 2-dichlorobenzene or so out of these two dichlorobenzenes, one which has the substitutions on one and fourth position, that is, the para positions will be the one that is formed as the major product okay.
or so out of these two dichlorobenzenes, one which has the substitutions on one and fourth position, that is, the para positions will be the one that is formed as the major product okay.
 So, the second reaction we will talk about is nitration reaction, so nitration reaction is by which we put a nitro group on to the aromatic ring and normally depending on the electron richness of the aromatic compound that is subjected to nitration we can use various reagents, so in this case you see that we use a reagent that is HNO3, nitric acid and sulfuric acid (H2SO4). A mixture of nitric acid (HNO3) and sulfuric acid (H2SO4) is sometimes called as a nitrating mixture, so this particular mixture will be able to nitrate a molecule because under these conditions, HNO3 gets protonated, and we would generate an electrophile which is NO+. So, this is the electrophile that is reacting, and this electrophile can then go on to ortho position or para-position forming two different mono nitro compounds, so 1-chloro-4-nitro benzene
So, the second reaction we will talk about is nitration reaction, so nitration reaction is by which we put a nitro group on to the aromatic ring and normally depending on the electron richness of the aromatic compound that is subjected to nitration we can use various reagents, so in this case you see that we use a reagent that is HNO3, nitric acid and sulfuric acid (H2SO4). A mixture of nitric acid (HNO3) and sulfuric acid (H2SO4) is sometimes called as a nitrating mixture, so this particular mixture will be able to nitrate a molecule because under these conditions, HNO3 gets protonated, and we would generate an electrophile which is NO+. So, this is the electrophile that is reacting, and this electrophile can then go on to ortho position or para-position forming two different mono nitro compounds, so 1-chloro-4-nitro benzene and 1-chloro-2-nitro benzene
and 1-chloro-2-nitro benzene . So, these are the two compounds that we would get and, if you have to say which is the major compound, because the major compound is the one where substitution is at the fourth position, we have already seen why. So, this is also a useful reaction of haloarenes.
Now the third reaction is sulfonation, so in sulfonation, what we add is a SO3H group, so it is called sulfonic acid group. So, if you take a haloarene and treat with concentrated H2SO4, so concentrate H2SO4 there again what happens is the H2SO4 molecule will protonate another H2SO4 molecules, and a water molecule leaves and effectively we get an electrophile which can be written like this. So, SO3H is the electrophile in these cases. So, then SO3H would react with the haloarene, both in ortho and para positions. We would get two products that is 4-chloro benzene, sulfonic acid
. So, these are the two compounds that we would get and, if you have to say which is the major compound, because the major compound is the one where substitution is at the fourth position, we have already seen why. So, this is also a useful reaction of haloarenes.
Now the third reaction is sulfonation, so in sulfonation, what we add is a SO3H group, so it is called sulfonic acid group. So, if you take a haloarene and treat with concentrated H2SO4, so concentrate H2SO4 there again what happens is the H2SO4 molecule will protonate another H2SO4 molecules, and a water molecule leaves and effectively we get an electrophile which can be written like this. So, SO3H is the electrophile in these cases. So, then SO3H would react with the haloarene, both in ortho and para positions. We would get two products that is 4-chloro benzene, sulfonic acid and 2-chlorobenzene sulfonic acid
and 2-chlorobenzene sulfonic acid , and out of these two structures, you already know that the 4-chlorobenzene sulfonic acid
, and out of these two structures, you already know that the 4-chlorobenzene sulfonic acid - would be the major product and 2-chlorobenzene sulfonic acid
- would be the major product and 2-chlorobenzene sulfonic acid would be the minor compound. Ok,
would be the minor compound. Ok,
 So, the next reaction that we will discuss is Friedel crafts alkylation. You already learned Friedel crafts alkylation of aromatic compounds. So, in this what is required is a haloalkane. So, we take a haloalkane and treat it with anhydrous AlCl3 aluminum chloride, where aluminum chloride (AlCl3) acts as a lewis acid and breaks the carbon CH3Cl bond so effectively. What we would have been something that can be represented as CH3+, especially if the alkyl halide used is methyl chloride
So, the next reaction that we will discuss is Friedel crafts alkylation. You already learned Friedel crafts alkylation of aromatic compounds. So, in this what is required is a haloalkane. So, we take a haloalkane and treat it with anhydrous AlCl3 aluminum chloride, where aluminum chloride (AlCl3) acts as a lewis acid and breaks the carbon CH3Cl bond so effectively. What we would have been something that can be represented as CH3+, especially if the alkyl halide used is methyl chloride . Then we do not really make a CH3+, but we would have something that is partially bonded to chlorine and having a lot of positive charge on CH3, so this again then gets bonded to aluminum. So, this is how we get we polarize this molecule and negative charge starts to develop on chlorine. So, we have an electrophile that is now an alkyl cation, a carbocation, and that electrophile would react with the haloalkane on ortho and para position and giving us 1-chloro-4-methyl benzene
. Then we do not really make a CH3+, but we would have something that is partially bonded to chlorine and having a lot of positive charge on CH3, so this again then gets bonded to aluminum. So, this is how we get we polarize this molecule and negative charge starts to develop on chlorine. So, we have an electrophile that is now an alkyl cation, a carbocation, and that electrophile would react with the haloalkane on ortho and para position and giving us 1-chloro-4-methyl benzene and 1-chloro-2-methyl benzene
and 1-chloro-2-methyl benzene , the two substituted product, the ortho substituted product - is the minor product.
This reaction has an interesting fact that, once we add an alkyl group onto a benzene ring onto an aromatic ring, the alkyl group makes the benzene ring more electron rich. So, there is only one problem with this reaction. Normally, when we carry out this reaction, the products that are formed in the reaction are more electron rich, are more activated than the starting materials themselves, so they might start giving you multiple alkylation. So, the reaction may not stop at forming one CH3. We might end up getting additional CH3 groups on aromatic ring, so this is one of the problems with Friedel Crafts alkylation, because the product always is more reactive than the starting material. So, that is something that we need to keep in mind whenever we want to do a Friedel craft alkylation. There are other problems with the reaction too, which you might learn if you take up chemistry in the higher class. Ok.
Now Friedel-Crafts acylation is another reaction where, instead of an alkyl halide, we use an acyl halide. So, these are acid chlorides. So, what I have here is acetyl chloride
, the two substituted product, the ortho substituted product - is the minor product.
This reaction has an interesting fact that, once we add an alkyl group onto a benzene ring onto an aromatic ring, the alkyl group makes the benzene ring more electron rich. So, there is only one problem with this reaction. Normally, when we carry out this reaction, the products that are formed in the reaction are more electron rich, are more activated than the starting materials themselves, so they might start giving you multiple alkylation. So, the reaction may not stop at forming one CH3. We might end up getting additional CH3 groups on aromatic ring, so this is one of the problems with Friedel Crafts alkylation, because the product always is more reactive than the starting material. So, that is something that we need to keep in mind whenever we want to do a Friedel craft alkylation. There are other problems with the reaction too, which you might learn if you take up chemistry in the higher class. Ok.
Now Friedel-Crafts acylation is another reaction where, instead of an alkyl halide, we use an acyl halide. So, these are acid chlorides. So, what I have here is acetyl chloride , so, we can call it the Friedel-Crafts + acetylation in this particular example. So, if you take acetyl halide, this also has a carbon-chlorine bond, and we use the same catalyst so the catalyst we use is anhydrous aluminum chloride (AlCl3). Now what would aluminum chloride (AlCl3) do? Aluminum chloride (AlCl3) would break the bond here and then we get CH3CO as the electrophile, so you would find that CH3CO with the positive charge on carbon, which would also be shared with oxygen. This is a relatively stable electrophile quite unlike methyl cation. So, this acyl cation would now be able to act as an electrophile and react with aromatic ring, giving us two products, two mono substituted products, one where the substitution is in fourth position, another one where the substitution is in second position. Of course, the major product is one where the substitution is in the fourth or para position.
Now, unlike alkylation reactions, acylation reactions would stop at mono substitution, because acyl group, because the product that is formed in this case, is a ketone, and once you have A CH3CO attached to benzene, it is called acetophenone
, so, we can call it the Friedel-Crafts + acetylation in this particular example. So, if you take acetyl halide, this also has a carbon-chlorine bond, and we use the same catalyst so the catalyst we use is anhydrous aluminum chloride (AlCl3). Now what would aluminum chloride (AlCl3) do? Aluminum chloride (AlCl3) would break the bond here and then we get CH3CO as the electrophile, so you would find that CH3CO with the positive charge on carbon, which would also be shared with oxygen. This is a relatively stable electrophile quite unlike methyl cation. So, this acyl cation would now be able to act as an electrophile and react with aromatic ring, giving us two products, two mono substituted products, one where the substitution is in fourth position, another one where the substitution is in second position. Of course, the major product is one where the substitution is in the fourth or para position.
Now, unlike alkylation reactions, acylation reactions would stop at mono substitution, because acyl group, because the product that is formed in this case, is a ketone, and once you have A CH3CO attached to benzene, it is called acetophenone . You will learn that while you study ketones, these compounds are more deactivated than haloarenes by themselves, so because an acetyl group and an acyl group normally deactivate the aromatic ring. So, therefore, the action would stop at one step, so in that way they would give you better control over an alkylation reaction. In both cases, aluminum chloride (AlCl3) is the lewis acid that is most commonly used.
There is only one issue: normally in Friedel-Crafts alkylation reaction. You would only need to use one equivalent of aluminum chloride (AlCl3) as the lewis acid, whereas in this you would only require to use aluminum chloride (AlCl3) in catalytic amount, because that is a catalyst that will continue to activate methyl chloride
. You will learn that while you study ketones, these compounds are more deactivated than haloarenes by themselves, so because an acetyl group and an acyl group normally deactivate the aromatic ring. So, therefore, the action would stop at one step, so in that way they would give you better control over an alkylation reaction. In both cases, aluminum chloride (AlCl3) is the lewis acid that is most commonly used.
There is only one issue: normally in Friedel-Crafts alkylation reaction. You would only need to use one equivalent of aluminum chloride (AlCl3) as the lewis acid, whereas in this you would only require to use aluminum chloride (AlCl3) in catalytic amount, because that is a catalyst that will continue to activate methyl chloride or the haloalkane that is used. But whereas whenever we do acylation reactions, the product has a keto group which coordinates with aluminum chloride (AlCl3). So, therefore, in these reactions, the amount of catalyst used is more, so you would have to use at least one equivalent of the catalyst for this reaction to go well, ok.
or the haloalkane that is used. But whereas whenever we do acylation reactions, the product has a keto group which coordinates with aluminum chloride (AlCl3). So, therefore, in these reactions, the amount of catalyst used is more, so you would have to use at least one equivalent of the catalyst for this reaction to go well, ok.
 So that is about the electrophilic substitution reactions of aromatic compounds. Now we would go ahead with the third kind of reaction that is reaction with metals, so this is probably one reaction where haloarenes exactly matches haloalkane. So, the reactivity pattern is not largely different, because you know in these reactions the halo compound is reacting with the metal and metals are substantially more electron electropositive than carbon itself, so they have similar pattern of reacting between haloalkanes and haloarenes. So, one of the reactions is called the Wurtz-Fitting reaction in this reaction. We can take a haloarene and a haloalkane and treat it with sodium and get these compounds which are cross coupled products where an alkyl group is now attached to an aryl. So, we get an R-Aryl, also an alkyl-aryl compound, sometimes called as aryl-alkyl compounds, so this can be prepared once there is a cross coupling.
Of course, there are problems with this reaction. As you can see, we could assume that two R groups can combine and give you a hydrocarbon, give you an alkyl, will give you an alkane. Similarly, two aromatic compounds can combine together to give you two aromatic rings linked with each other through a single bond, so that is possible, and that reaction is called a fitting reaction. So, in fitting reaction, what happens is two halo alkenes react together in the presence of sodium. Two molecules of sodium halide come out, and then we get a compound where two aromatic rings are linked together through a single bond. These kinds of compounds are called bi aryl and in this particular example, we have two phenyl rings which are linked together, and this is called biphenyl. So, we can prepare biphenyl by using this reaction, but it is you would normally feel that the synthetic utility is not much because we are using metallic sodium for this reaction, so metallic sodium is very reactive, and it would normally catch fire if you were not careful and so on. It reacts violently with water moisture. So, therefore, the moisture in the air is sufficient to give you a very explosive reaction. So, therefore this is practically not used much, but we have to realize that this is a theoretical possibility. This is something that we can do while studying the reactions of alkyl halides with metals. You have studied Wurtz reaction, so Wurtz reaction was when an alkyl halide is treated with sodium to get a dialkyl compound, dialkyl hydrocarbon. Now, once you do and once you do the same reaction with haloarenes, we call it fitting. So, that is why this reaction, where it’s actually a mixture of Fitting reaction and Wurtz reaction, is called Wurtz-fitting reaction. So, the name will make sense to you if you start looking at this compound.
So, this is pretty much about the reactions of haloarenes. So, now we have discussed all the three kinds of reactions that haloarenes can give you, so the reactions of haloarenes are electrophilic substitution reactions, which are probably the most useful, nucleophilic substitutional reactions through Misenheimer complexes, not so much useful, but it does happen. And finally, the reaction with metals, where we can do this coupling reactions either fitting reactions or Wurtz-fitting reactions.
So that is about the electrophilic substitution reactions of aromatic compounds. Now we would go ahead with the third kind of reaction that is reaction with metals, so this is probably one reaction where haloarenes exactly matches haloalkane. So, the reactivity pattern is not largely different, because you know in these reactions the halo compound is reacting with the metal and metals are substantially more electron electropositive than carbon itself, so they have similar pattern of reacting between haloalkanes and haloarenes. So, one of the reactions is called the Wurtz-Fitting reaction in this reaction. We can take a haloarene and a haloalkane and treat it with sodium and get these compounds which are cross coupled products where an alkyl group is now attached to an aryl. So, we get an R-Aryl, also an alkyl-aryl compound, sometimes called as aryl-alkyl compounds, so this can be prepared once there is a cross coupling.
Of course, there are problems with this reaction. As you can see, we could assume that two R groups can combine and give you a hydrocarbon, give you an alkyl, will give you an alkane. Similarly, two aromatic compounds can combine together to give you two aromatic rings linked with each other through a single bond, so that is possible, and that reaction is called a fitting reaction. So, in fitting reaction, what happens is two halo alkenes react together in the presence of sodium. Two molecules of sodium halide come out, and then we get a compound where two aromatic rings are linked together through a single bond. These kinds of compounds are called bi aryl and in this particular example, we have two phenyl rings which are linked together, and this is called biphenyl. So, we can prepare biphenyl by using this reaction, but it is you would normally feel that the synthetic utility is not much because we are using metallic sodium for this reaction, so metallic sodium is very reactive, and it would normally catch fire if you were not careful and so on. It reacts violently with water moisture. So, therefore, the moisture in the air is sufficient to give you a very explosive reaction. So, therefore this is practically not used much, but we have to realize that this is a theoretical possibility. This is something that we can do while studying the reactions of alkyl halides with metals. You have studied Wurtz reaction, so Wurtz reaction was when an alkyl halide is treated with sodium to get a dialkyl compound, dialkyl hydrocarbon. Now, once you do and once you do the same reaction with haloarenes, we call it fitting. So, that is why this reaction, where it’s actually a mixture of Fitting reaction and Wurtz reaction, is called Wurtz-fitting reaction. So, the name will make sense to you if you start looking at this compound.
So, this is pretty much about the reactions of haloarenes. So, now we have discussed all the three kinds of reactions that haloarenes can give you, so the reactions of haloarenes are electrophilic substitution reactions, which are probably the most useful, nucleophilic substitutional reactions through Misenheimer complexes, not so much useful, but it does happen. And finally, the reaction with metals, where we can do this coupling reactions either fitting reactions or Wurtz-fitting reactions.
 So, in the last part of this chapter what we would discuss is about some poly halogen compounds that are most commonly found and most commonly used, so the first member. So, these are poly halogen compounds. That means these are compounds where at least two halogen atoms are attached to the carbon atom, so the simplest member is that that you would come across is dichloromethane
So, in the last part of this chapter what we would discuss is about some poly halogen compounds that are most commonly found and most commonly used, so the first member. So, these are poly halogen compounds. That means these are compounds where at least two halogen atoms are attached to the carbon atom, so the simplest member is that that you would come across is dichloromethane dichloromethane is a liquid. So, if you take it at room temperature, it has a boiling point around 40 degrees, so it is a liquid but volatile liquid. So, if you keep it just vanishes and it is normally used as a solvent in organic chemistry labs wherever people use it, it is as a solvent in industry. It can be used as a paint remover because most of the paints are organic compounds and because dichloromethane
dichloromethane is a liquid. So, if you take it at room temperature, it has a boiling point around 40 degrees, so it is a liquid but volatile liquid. So, if you keep it just vanishes and it is normally used as a solvent in organic chemistry labs wherever people use it, it is as a solvent in industry. It can be used as a paint remover because most of the paints are organic compounds and because dichloromethane is a solvent for organic compounds, it can be used to remove those and because it is volatile it vaporizes faster, so it can also be used as a propellant in aerosol. Now, however, it is not a nice compound to meddle around with because it harms if you inhale and see it has a low boiling point. So, if you keep a bottle of dichloromethane
is a solvent for organic compounds, it can be used to remove those and because it is volatile it vaporizes faster, so it can also be used as a propellant in aerosol. Now, however, it is not a nice compound to meddle around with because it harms if you inhale and see it has a low boiling point. So, if you keep a bottle of dichloromethane open in this room after some time you would have dichloromethane fumes in the room, and it harms the human central nervous system. So, therefore, it is not good if you are subjected to this compound, and one other thing is that whenever we use them in labs, if it happens to fall on your body and the hand especially and this part of the skin, that is more sensitive, like between Your fingers, for example, and between the nails and so on, you will immediately start to feel an extremely burning sensation. So, dichloromethane
open in this room after some time you would have dichloromethane fumes in the room, and it harms the human central nervous system. So, therefore, it is not good if you are subjected to this compound, and one other thing is that whenever we use them in labs, if it happens to fall on your body and the hand especially and this part of the skin, that is more sensitive, like between Your fingers, for example, and between the nails and so on, you will immediately start to feel an extremely burning sensation. So, dichloromethane has this problem, so it probably absorbs through the skin too. So, if it touches your skin on, especially on sensitive skin, it will start to give you a very burning sensation. So, these are things that we should be careful of when we deal with dichloromethane
has this problem, so it probably absorbs through the skin too. So, if it touches your skin on, especially on sensitive skin, it will start to give you a very burning sensation. So, these are things that we should be careful of when we deal with dichloromethane , but, however, its applications are so good. It is a very good solvent that is still being continuously used, especially in organic chemistry laboratories. Now the next compound is trichloromethane
, but, however, its applications are so good. It is a very good solvent that is still being continuously used, especially in organic chemistry laboratories. Now the next compound is trichloromethane , which you would all know more of as a chloroform. Now chloroform
, which you would all know more of as a chloroform. Now chloroform again, is a solvent. It is a very good solvent for fats. All sorts of fats can be dissolved in it and are used as a solvent for alkaloids. So, like you know, alkaloids are compounds that contain nitrogen atoms, so these are natural products which are available in nature, so whenever we would like to extract them from natural sources, so imagine that there is a biologically active compound present in a plant material and if you would like to extract it. Chloroform
again, is a solvent. It is a very good solvent for fats. All sorts of fats can be dissolved in it and are used as a solvent for alkaloids. So, like you know, alkaloids are compounds that contain nitrogen atoms, so these are natural products which are available in nature, so whenever we would like to extract them from natural sources, so imagine that there is a biologically active compound present in a plant material and if you would like to extract it. Chloroform is one of the solvents that you can use to extract out, alkaloids it also dissolves iodine, bromine and so on. Now it is also used for the production of refrigerant freon R-22
is one of the solvents that you can use to extract out, alkaloids it also dissolves iodine, bromine and so on. Now it is also used for the production of refrigerant freon R-22 . So, R-22 is the compound that is attached to CH, so the compound has, it is a fluoro. So, freons are all we will talk about them. Freons are all compounds that have fluorine and chlorine attached to the same carbon atom. Now, if you take methane, add two fluorine, one chlorine and one hydrogen, then that compound is called R-22
. So, R-22 is the compound that is attached to CH, so the compound has, it is a fluoro. So, freons are all we will talk about them. Freons are all compounds that have fluorine and chlorine attached to the same carbon atom. Now, if you take methane, add two fluorine, one chlorine and one hydrogen, then that compound is called R-22 , so, this is made from chloroform
, so, this is made from chloroform , so that is one of its applications. It has an anesthetic effect. So, if you inhale it, you will feel dizzy, so it has an anesthetic effect. So, just like dichloromethane
, so that is one of its applications. It has an anesthetic effect. So, if you inhale it, you will feel dizzy, so it has an anesthetic effect. So, just like dichloromethane , it is harmful, so you cannot inhale it too much if inhaling them in smaller amounts will start to feel dizzy. So, it has an anesthetic effect and what is more damaging is if we get continuously, if we inhale a lot of it, it can damage our liver and kidney so because in the liver chloroform
, it is harmful, so you cannot inhale it too much if inhaling them in smaller amounts will start to feel dizzy. So, it has an anesthetic effect and what is more damaging is if we get continuously, if we inhale a lot of it, it can damage our liver and kidney so because in the liver chloroform starts to get processed, so liver takes care of all these bad compounds. That gets into your body, so it will start to process chloroform and start to generate phosgene
starts to get processed, so liver takes care of all these bad compounds. That gets into your body, so it will start to process chloroform and start to generate phosgene in liver and all these byproducts that are formed they can also harm your kidney, so chloroform
in liver and all these byproducts that are formed they can also harm your kidney, so chloroform is not something that we should be inhaling. It is also oxidized by air in the presence of light, so if there is light present, so you know this carbon-chlorine bonds can break because they we have seen that before that carbon-chlorine bonds are weak, so sometimes they get broken if energy is provided in the form of light. So, if there is oxygen and air present, then chloroform would break down into a compound that is called phosgene
is not something that we should be inhaling. It is also oxidized by air in the presence of light, so if there is light present, so you know this carbon-chlorine bonds can break because they we have seen that before that carbon-chlorine bonds are weak, so sometimes they get broken if energy is provided in the form of light. So, if there is oxygen and air present, then chloroform would break down into a compound that is called phosgene . So, I would show the structure of phosgene
. So, I would show the structure of phosgene here, so chloroform
here, so chloroform would break down into phosgene and phosgene is an extremely toxic compound. So, if you inhale toxic phosgene
would break down into phosgene and phosgene is an extremely toxic compound. So, if you inhale toxic phosgene death is a sure thing if you, if we inhale them in larger amounts, so chloroform
death is a sure thing if you, if we inhale them in larger amounts, so chloroform itself is harmful, but phosgene
itself is harmful, but phosgene is toxic by its just, not harmful. It is toxic, but it has a nice smell. It has a smell. That is similar to the fruit sapota. If you know Chikku that is called in Hindi, so this has a very pleasant smell, but it is an extremely toxic compound, so we should be very careful while handling chloroform
is toxic by its just, not harmful. It is toxic, but it has a nice smell. It has a smell. That is similar to the fruit sapota. If you know Chikku that is called in Hindi, so this has a very pleasant smell, but it is an extremely toxic compound, so we should be very careful while handling chloroform . So, therefore chloroform is not normally kept in a bottle half filled, because if you take a bottle and fill chloroform to the half, the remaining half is air. So, now, if this is subjected to, this is exposed to light then phosgene generates and phosgene
. So, therefore chloroform is not normally kept in a bottle half filled, because if you take a bottle and fill chloroform to the half, the remaining half is air. So, now, if this is subjected to, this is exposed to light then phosgene generates and phosgene is gas. So, therefore, whoever opens the bottle would actually get exposed to phosgene. So, therefore, normally chloroform
is gas. So, therefore, whoever opens the bottle would actually get exposed to phosgene. So, therefore, normally chloroform is always kept in dark, colored bottles and as much as possible. We fill it to the top so that there is no air present to react with chloroform now.
The third compound I will talk about is triiodomethane
is always kept in dark, colored bottles and as much as possible. We fill it to the top so that there is no air present to react with chloroform now.
The third compound I will talk about is triiodomethane , so, this is similar to chloroform
, so, this is similar to chloroform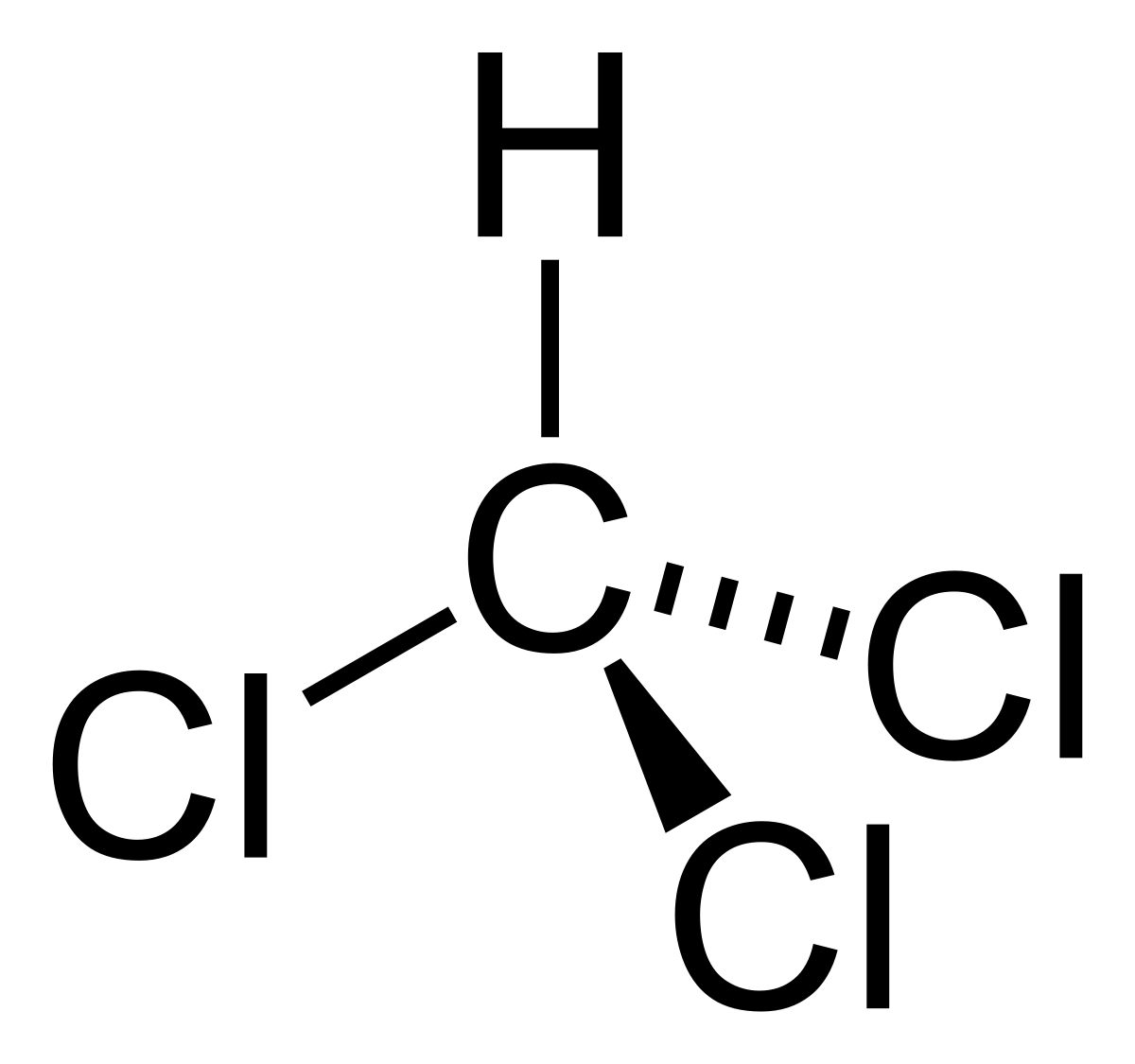 . The chlorine is replaced with iodine, so it is CHI3. Iodoform
. The chlorine is replaced with iodine, so it is CHI3. Iodoform was earlier used as a source of iodine. So, an iodine is known to have a very good effect because it can kill a lot of microorganisms, so it is used for healing wounds and so on. So, it was used as an antiseptic. The reason why it was being used, even without knowing people used to apply iodoform and iodoform
was earlier used as a source of iodine. So, an iodine is known to have a very good effect because it can kill a lot of microorganisms, so it is used for healing wounds and so on. So, it was used as an antiseptic. The reason why it was being used, even without knowing people used to apply iodoform and iodoform when exposed generates iodine, so it was actually iodine that was acting as the antiseptic so when later on realizing that it is just iodine that is doing it. Now iodoform
when exposed generates iodine, so it was actually iodine that was acting as the antiseptic so when later on realizing that it is just iodine that is doing it. Now iodoform is replaced with other compounds, but earlier it was used as an antiseptic.
is replaced with other compounds, but earlier it was used as an antiseptic.
 Now the next compound we will talk about is tetrachloromethane CCl4 or carbon tetrachloride
Now the next compound we will talk about is tetrachloromethane CCl4 or carbon tetrachloride carbon attached to four chlorine atoms. This is used for the preparation of a lot of refrigerants and because it can be used for making freon that I have already talked to you about, and they can also be used as propellants. It has a boiling point of around 75 degrees, so it can be used also, and it gives you vapors now the problem with carbon tetrachloride
carbon attached to four chlorine atoms. This is used for the preparation of a lot of refrigerants and because it can be used for making freon that I have already talked to you about, and they can also be used as propellants. It has a boiling point of around 75 degrees, so it can be used also, and it gives you vapors now the problem with carbon tetrachloride is it not advisable to use them not even as much as chloroform
is it not advisable to use them not even as much as chloroform or dichloromethane
or dichloromethane , because it is known to cause damage to nerve cells, and it can also cause liver cancer in humans, so it might cause, depending on the level of exposure, it may cause liver cancer. So, therefore, carbon tetrachloride
, because it is known to cause damage to nerve cells, and it can also cause liver cancer in humans, so it might cause, depending on the level of exposure, it may cause liver cancer. So, therefore, carbon tetrachloride , we have to be extremely careful with and another problem is carbon tetrachloride if it is released into the atmosphere, it just moves up and reaches to the top and interacts with the ozone layer, and then it depletes the ozone layer through a free radical reaction where the carbon-chlorine bond breaks and the radical formed would start to react with the ozone thereby depleting ozone and thus causing problem, okay.
Now so we saw for we have been saying that chloroform
, we have to be extremely careful with and another problem is carbon tetrachloride if it is released into the atmosphere, it just moves up and reaches to the top and interacts with the ozone layer, and then it depletes the ozone layer through a free radical reaction where the carbon-chlorine bond breaks and the radical formed would start to react with the ozone thereby depleting ozone and thus causing problem, okay.
Now so we saw for we have been saying that chloroform and carbon tetrachloride
and carbon tetrachloride can be used for making freons. So, these freons are, as I have told you once - they are compounds that are linked to fluorine and chlorine, so they can also be called chlorofluorocarbon compounds. So, these are compounds where a carbon atom is attached to chlorine and fluorine and may be additional carbon-carbon bonds. They are stable. These compounds are extremely stable. They are unreactive, they do not normally react, they are non-corrosive. They do not cause any corrosion by themselves, and they are gases, but they can easily be liquefied because they are high-density gases, so they can be liquefied by applying pressure now. Freon 12 or CCl2F2
can be used for making freons. So, these freons are, as I have told you once - they are compounds that are linked to fluorine and chlorine, so they can also be called chlorofluorocarbon compounds. So, these are compounds where a carbon atom is attached to chlorine and fluorine and may be additional carbon-carbon bonds. They are stable. These compounds are extremely stable. They are unreactive, they do not normally react, they are non-corrosive. They do not cause any corrosion by themselves, and they are gases, but they can easily be liquefied because they are high-density gases, so they can be liquefied by applying pressure now. Freon 12 or CCl2F2  is one of the most used in industries and it is prepared by you from carbon tetrachloride
is one of the most used in industries and it is prepared by you from carbon tetrachloride using Swartz reaction. So, Swartz reaction is something we learned. We learned whenever we have to make a fluoroalkane, we take a chloroalkane or a bromo alkene and treat it with silver fluoride(AgF) or cobalt fluoride (CoF2) and so on. Certain metal fluorides, which would then precipitate a metal chloride or a metal bromide and form these carbon-fluorine bonds. So, the Swartz reaction is used for making freons from compounds that have carbon-chlorine bonds. Now they again are used as aerosols, propellants, refrigerants and for air conditions, and so on. Freons, just like carbon tetrachloride
using Swartz reaction. So, Swartz reaction is something we learned. We learned whenever we have to make a fluoroalkane, we take a chloroalkane or a bromo alkene and treat it with silver fluoride(AgF) or cobalt fluoride (CoF2) and so on. Certain metal fluorides, which would then precipitate a metal chloride or a metal bromide and form these carbon-fluorine bonds. So, the Swartz reaction is used for making freons from compounds that have carbon-chlorine bonds. Now they again are used as aerosols, propellants, refrigerants and for air conditions, and so on. Freons, just like carbon tetrachloride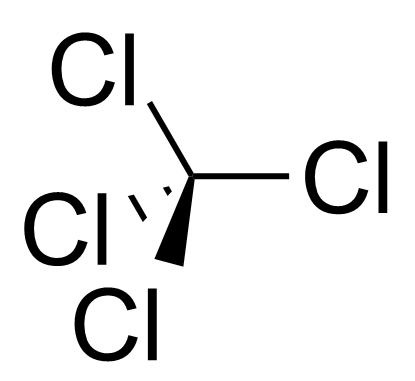 , are one of the major causes of the depletion of ozone layer, because freons would again move up the atmosphere reach where the ozone layer is and then once it reaches there. It would start to react with ozone through free, radical that are generated from these freons and therefore deplete the ozone layer which would result in ultraviolet radiations coming through the atmosphere into earth and would affect all the living organisms because we cannot be exposed to ultraviolet radiation, so this is one of the disadvantages of using freon. So, no matter how carefully, we use crayons at some point in time. They are going to be released into the atmosphere and finally, they are going to result in the depletion of the ozone layer. So, this is something that we should take care of and try to avoid the use of freons with other refrigerants, for example, where the use of these harmful chemicals can be avoided.
, are one of the major causes of the depletion of ozone layer, because freons would again move up the atmosphere reach where the ozone layer is and then once it reaches there. It would start to react with ozone through free, radical that are generated from these freons and therefore deplete the ozone layer which would result in ultraviolet radiations coming through the atmosphere into earth and would affect all the living organisms because we cannot be exposed to ultraviolet radiation, so this is one of the disadvantages of using freon. So, no matter how carefully, we use crayons at some point in time. They are going to be released into the atmosphere and finally, they are going to result in the depletion of the ozone layer. So, this is something that we should take care of and try to avoid the use of freons with other refrigerants, for example, where the use of these harmful chemicals can be avoided.
 The last compound, the last poly halogen compound that I would talk about is probably the most talked about talked about among all the poly halogen commons. This is DDT, so the structure of DDT
The last compound, the last poly halogen compound that I would talk about is probably the most talked about talked about among all the poly halogen commons. This is DDT, so the structure of DDT is given here, so you can see the structure of DDT in this slide. So, this has a trichloromethyl group and there is another carbon, so this is trichloroethane and the second carbon atom is attached to two benzene rings which are substituted with chlorine atom. So, one of the ways of naming it is p, p’ that means p, p’-dichlorophenyl trichloroethane
is given here, so you can see the structure of DDT in this slide. So, this has a trichloromethyl group and there is another carbon, so this is trichloroethane and the second carbon atom is attached to two benzene rings which are substituted with chlorine atom. So, one of the ways of naming it is p, p’ that means p, p’-dichlorophenyl trichloroethane . So, we have chlorophenyl groups, two of them, so that is we say, dichlorophenyl and then trichloroethane. So, this part of the molecule is trichloride part. So, this is DDT, so DDT was known for a long time, but in the 1930s it there was a scientist called Paul Hermann Muller, who found out that this particular compound has, can kill a lot of insects. It can kill a number of arthropods. So, suddenly, this started to be used as an insecticide. It started to be used as a pesticide in agriculture in households. People started to use this and so on, and this discovery was so important at that point, because there were various diseases that were spreading through the human population by way of insects. So, it included diseases like malaria, which is spread by mosquitoes. So, that is one of the examples. So, in order to prevent the spread of these releases, these diseases people started to use DDT
. So, we have chlorophenyl groups, two of them, so that is we say, dichlorophenyl and then trichloroethane. So, this part of the molecule is trichloride part. So, this is DDT, so DDT was known for a long time, but in the 1930s it there was a scientist called Paul Hermann Muller, who found out that this particular compound has, can kill a lot of insects. It can kill a number of arthropods. So, suddenly, this started to be used as an insecticide. It started to be used as a pesticide in agriculture in households. People started to use this and so on, and this discovery was so important at that point, because there were various diseases that were spreading through the human population by way of insects. So, it included diseases like malaria, which is spread by mosquitoes. So, that is one of the examples. So, in order to prevent the spread of these releases, these diseases people started to use DDT in larger amounts and started spreading them, so it was at that time such a find. It was such a useful compound that Mueller got Nobel prize in 1948 for finding the biological finding the application of DDT, so it was talked about so much and its use was so much that people started to use it.
But there was a problem associated with DDT
in larger amounts and started spreading them, so it was at that time such a find. It was such a useful compound that Mueller got Nobel prize in 1948 for finding the biological finding the application of DDT, so it was talked about so much and its use was so much that people started to use it.
But there was a problem associated with DDT . The DDT once it goes into the environment, it does not get disintegrated. So, therefore, what happens is once it is sprayed in a field in an agricultural field or something it just gets washed down into the water bodies and every fish and other animals that are living in the water body would start to consume compounds or would get affected by DDT
. The DDT once it goes into the environment, it does not get disintegrated. So, therefore, what happens is once it is sprayed in a field in an agricultural field or something it just gets washed down into the water bodies and every fish and other animals that are living in the water body would start to consume compounds or would get affected by DDT  or they will go into the body of these animals from water and after some time these fish will be consumed by bigger animals such as birds, for example, and then DDT gets into the body of birds. So, a bird will consume a very large number of fishes and because DDT does not get out of the body or does not get disintegrated. Therefore, the amount of DDT
or they will go into the body of these animals from water and after some time these fish will be consumed by bigger animals such as birds, for example, and then DDT gets into the body of birds. So, a bird will consume a very large number of fishes and because DDT does not get out of the body or does not get disintegrated. Therefore, the amount of DDT  present in the animal gets increased over a period of time and this results in various problems. So, for birds, one of the big issues was that the eggshells of many birds, including eagles, pelicans and so on, started to become extremely weaker and crumbled and therefore the eggs were never hatching.
So, this resulted in a lot of issues and by the 1960s people started to realize that use of DDT has to be avoided somehow, so there was a big protest against the production and use of DDT
present in the animal gets increased over a period of time and this results in various problems. So, for birds, one of the big issues was that the eggshells of many birds, including eagles, pelicans and so on, started to become extremely weaker and crumbled and therefore the eggs were never hatching.
So, this resulted in a lot of issues and by the 1960s people started to realize that use of DDT has to be avoided somehow, so there was a big protest against the production and use of DDT . Therefore, by 1972 DDT was banned from agricultural applications in U.S and in 1973 the government approved that this decision is good and from 1973 onwards in the United States of America DDT is not being used. However, they continued to produce it until 1986. They were producing, DDT and selling it to other countries, and countries, including India, are still using DDT and right now India is the only country that produces DDT, so even China has stopped producing it now, but India still produces DDT. The harmful effects of DDT
. Therefore, by 1972 DDT was banned from agricultural applications in U.S and in 1973 the government approved that this decision is good and from 1973 onwards in the United States of America DDT is not being used. However, they continued to produce it until 1986. They were producing, DDT and selling it to other countries, and countries, including India, are still using DDT and right now India is the only country that produces DDT, so even China has stopped producing it now, but India still produces DDT. The harmful effects of DDT are known, but still, it is sad thing that we are using it because it is an effective insecticide. So, any other replacement of DDT with other compounds is expensive. So, that is one of the reasons why DDT is continuing to be used, but this is something that has to be avoided if possible. So, in short, whenever we talk about poly halogen compound DDT
are known, but still, it is sad thing that we are using it because it is an effective insecticide. So, any other replacement of DDT with other compounds is expensive. So, that is one of the reasons why DDT is continuing to be used, but this is something that has to be avoided if possible. So, in short, whenever we talk about poly halogen compound DDT  is something that we cannot ignore. So, so that is a poly halogen compound that had a lot of application which people start stop to apply, and now we are, we have to reach a phase where we stop using DDT completely.
is something that we cannot ignore. So, so that is a poly halogen compound that had a lot of application which people start stop to apply, and now we are, we have to reach a phase where we stop using DDT completely.
 So, this is all about this particular unit. So, in this unit we have discussed the reactions of haloalkanes and haloarenes in detail, and you would know that the various topics that we covered, so we started off this unit by discussing about some very much biologically active compounds that fall into this category. At that time, itself, I told you that poly halogen compounds are harmful, so we have now seen some of the examples and you know, despite having certain applications, they are still harmful, so they can, they have to be used with care. Then we went ahead, and we discussed the classifications of haloalkanes and haloarenes. The simplest classification was as mono halogenated or poly halogenated compounds and so on. Then we discussed the methods of preparing them. Alkyl halides or haloalkanes are prepared largely from alcohols by using either HCl or you can use phosphorus trihalides (PX3) or phosphorus oxychloride (POCl3), and the best method to make chloroalkanes from alkyl halides is by using thionyl chloride (SOCl2). So, because the byproducts that were formed are gaseous, haloalkanes are prepared mostly by using electrophilic aromatic substitution and also by way of Sandmeyer reaction, which we have seen. While most of the synthesis of these compounds would rely on the synthesis of chlorinated and brominated compounds. Fluorinated and iodinated compounds so fluoro and iodo Organo compounds are generally prepared by doing halogen exchange.
Then we went ahead and talked about the properties of these molecules, their physical properties. They generally have higher boiling points than their hydrocarbons. They are dense. Most of the poly halogenated compounds are denser than water, however, their solubility in water is very less so these are the main points that we discussed. Then coming to the chemical properties or reactivities of alkyl halides, alkyl halides have three major reactions. The most important one is nucleophilic substitution reactions, then the second one is elimination, reactions of haloalkanes, which gives you alkenes, and finally, the reaction of haloalkanes with metals, where Grignard reagent was one of the very useful reagents that we could prepare by making a carbon-magnesium bond, so we talked about that and you in later units you would see that Grignard reagent is used in organic synthesis for making very many number of compounds and in the reactions of haloarenes which we discussed today, we said that nucleophilic substitution reactions are possible, but under harsh conditions, but electrophilic substitution reactions of haloarenes are the most ah commonly discussed one. Haloarenes do not have an elimination reaction in general, because an elimination would require that you put a triple bond in an aromatic ring. So, haloarenes do not give you elimination reaction, except for under very special conditions, and they also do react with metals where but most of the reactions that they can also form Grignard reagents with metal when they are treated with magnesium. But we mostly discussed the fitting reaction and Wurtz-fitting reaction in this. And finally, we talked about poly halogen compounds, and we discussed a number of their applications. But we emphasized the fact that poly halogenated compounds cannot be used in larger amounts and as much as possible their application has to be replaced with certain other compounds because they might continue to be in the environment and cause harm to the living organisms. So, that is the end of this unit. Thank you very much.
So, this is all about this particular unit. So, in this unit we have discussed the reactions of haloalkanes and haloarenes in detail, and you would know that the various topics that we covered, so we started off this unit by discussing about some very much biologically active compounds that fall into this category. At that time, itself, I told you that poly halogen compounds are harmful, so we have now seen some of the examples and you know, despite having certain applications, they are still harmful, so they can, they have to be used with care. Then we went ahead, and we discussed the classifications of haloalkanes and haloarenes. The simplest classification was as mono halogenated or poly halogenated compounds and so on. Then we discussed the methods of preparing them. Alkyl halides or haloalkanes are prepared largely from alcohols by using either HCl or you can use phosphorus trihalides (PX3) or phosphorus oxychloride (POCl3), and the best method to make chloroalkanes from alkyl halides is by using thionyl chloride (SOCl2). So, because the byproducts that were formed are gaseous, haloalkanes are prepared mostly by using electrophilic aromatic substitution and also by way of Sandmeyer reaction, which we have seen. While most of the synthesis of these compounds would rely on the synthesis of chlorinated and brominated compounds. Fluorinated and iodinated compounds so fluoro and iodo Organo compounds are generally prepared by doing halogen exchange.
Then we went ahead and talked about the properties of these molecules, their physical properties. They generally have higher boiling points than their hydrocarbons. They are dense. Most of the poly halogenated compounds are denser than water, however, their solubility in water is very less so these are the main points that we discussed. Then coming to the chemical properties or reactivities of alkyl halides, alkyl halides have three major reactions. The most important one is nucleophilic substitution reactions, then the second one is elimination, reactions of haloalkanes, which gives you alkenes, and finally, the reaction of haloalkanes with metals, where Grignard reagent was one of the very useful reagents that we could prepare by making a carbon-magnesium bond, so we talked about that and you in later units you would see that Grignard reagent is used in organic synthesis for making very many number of compounds and in the reactions of haloarenes which we discussed today, we said that nucleophilic substitution reactions are possible, but under harsh conditions, but electrophilic substitution reactions of haloarenes are the most ah commonly discussed one. Haloarenes do not have an elimination reaction in general, because an elimination would require that you put a triple bond in an aromatic ring. So, haloarenes do not give you elimination reaction, except for under very special conditions, and they also do react with metals where but most of the reactions that they can also form Grignard reagents with metal when they are treated with magnesium. But we mostly discussed the fitting reaction and Wurtz-fitting reaction in this. And finally, we talked about poly halogen compounds, and we discussed a number of their applications. But we emphasized the fact that poly halogenated compounds cannot be used in larger amounts and as much as possible their application has to be replaced with certain other compounds because they might continue to be in the environment and cause harm to the living organisms. So, that is the end of this unit. Thank you very much.










