Chemistry Class 12 Unit 08 Chapter 01 D And F Block Elements L 1 5 Ljtjh5J Un0 En Punc Para Txt
Good morning, everybody. Today we will be starting on another chapter, which is d and f block elements. So, what are these elements, particularly? We should know, and what are those positions? Another name of these block elements is transition elements. So, one important definition for this is transition, so these are transition elements and if we consider their position in the periodic table, they run from group 3 to group 11 in the d block. We all know that when we start from the left-hand side of the periodic table, we find that group 1 and group 2 elements are there, and at some point, in time, we will find that group 3 up to group 11 come and these are transition elements. If they are present from the occupancy in the d shell, then we consider them as elements of d block, so we will just go up to group 11. So, what about group 12?
If we ask somebody what the group 12 elements are, give some examples of group 12 elements. Some can say that we have zinc, cadmium, and mercury, so that question immediately comes to us that, whether we should include group 12 within these or not? That we will discuss later. So, what about these (group 3 to 11 elements)? What is the transition, because of their position in the periodic table, i.e., they are present in between s block and the p block element, so their position in the periodic table is important in the periodic table. Here we have the s block elements and, on the right hand, side, we have the p block elements. So, positioning of these elements is therefore important, and this position is the transition position from s to p. If these are s and these are p block elements, we move for a transition from s to p via these elements.
 That is why these are known as transition elements and in terms of their properties, we will find that the properties are also transitional. So, what is the transitional property?
This means properties will be in between s block elements and the p block elements. The first thing that we consider is their metallic properties. So, what are the metallic properties of s and p block elements like sodium, potassium magnesium and calcium? We know that they are corresponding metallic in nature, so when we move from them to these elements, we will find that they are also highly reactive metallic elements. If we move from these, which is also very much similar to that of s block elements, as we slightly move from s to these, they will also form typically ionic compounds, and we know that the elements on the right hand, side, including the halogens, they form the corresponding elements in the p block, but these p block elements are largely covalent, so they will get some property inherited from these p block elements also - and in some cases in the latter part of the periodic table on the right hand, side of these d block elements they will also form some covalent characters. Something related to these p block elements. So, as now we know s block property is also there and so is the p-block. Elements of p block, as gives typically or covalent compounds. So, some of these transition elements also will be responsible for giving typically all these covalent characters to this series.
That is why these are known as transition elements and in terms of their properties, we will find that the properties are also transitional. So, what is the transitional property?
This means properties will be in between s block elements and the p block elements. The first thing that we consider is their metallic properties. So, what are the metallic properties of s and p block elements like sodium, potassium magnesium and calcium? We know that they are corresponding metallic in nature, so when we move from them to these elements, we will find that they are also highly reactive metallic elements. If we move from these, which is also very much similar to that of s block elements, as we slightly move from s to these, they will also form typically ionic compounds, and we know that the elements on the right hand, side, including the halogens, they form the corresponding elements in the p block, but these p block elements are largely covalent, so they will get some property inherited from these p block elements also - and in some cases in the latter part of the periodic table on the right hand, side of these d block elements they will also form some covalent characters. Something related to these p block elements. So, as now we know s block property is also there and so is the p-block. Elements of p block, as gives typically or covalent compounds. So, some of these transition elements also will be responsible for giving typically all these covalent characters to this series.
What we will find now that many of these properties, these two properties we mostly consider which are the physical properties of these elements and the chemical properties, since we are considering their properties in relation to s block and p block elements and what we are doing in these blocks, that we are adding electrons not on the ultimate or the outermost shell, but in the penultimate shell is responsible, so we have the penultimate shell and the shell is also expanded when we know that s is filled and p is filled. We get eight electrons, but in this case the d level is filled. D shell is filled, so we move from 8 to 18 electron occupancy. As a result, many of these physical and chemical properties of these metals, when you consider these as the metals they have the metallic property, so metals of these group, such as nickel, copper what are these metals, they will have some properties which are in common, and they give rise to something which is typically the metallic property. That means they are good conductors, such as, for both these two things. That means they are good conductors for electricity and heat. Then they can have metallic clusters. They are also hard and strong, because when you talk in terms of the metallurgical behavior of some of these metals such as iron, also comes into this category.
As the transition element of the d block element, how we can improve that? The property which is strongly related to the metallic property and in some cases, they are also ductile and another property which is very much related to their physical property, is that they also form alloys with other metals. So, these group of elements how we can define we just go for the definition, because the definition will consider all the species which will be there and will have if we have the d electron configuration. Whether that d electron configuration can have connection with this definition of these transition elements. We will see from here that, not only the d but also will be considering the f block elements in a similar fashion.
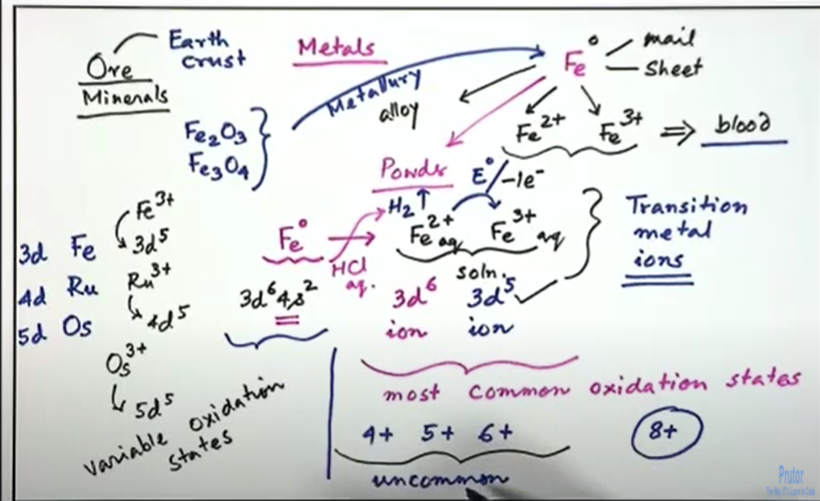
 In the latter part of this chapter, we will first find what are those f block elements so before that we will just consider what are these d elements and what are the transition metal ions. By definition, a transition metal is an element whose atom has partially filled d sub shell. So, the occupancy of these d sub shells is important and can give rise to cations with an incomplete d sub shell. If it can give rise to some cations, which have in corporately filled d sub shell, then that metal or that element we consider as the d block element. So, you have the incompletely filled d orbitals. If we have the d shell or the d orbitals, then we will find that these are all incompletely filled and where this incompletely filled d, orbitals, or d shell is in its ground state or in any one of its oxidation states, which is therefore important that the ground state configuration should give us an importantly filled d shell or any of its oxidation states, so when we consider their possibility of the oxidation states, whether it has a one permanent or one very easily accessible, oxidation state or it can have the different oxidation state. Whatever oxidation state, you can have, whether you have an incompletely filled d shell or not, that will typically define whether you are talking about the corresponding element, which is a transition element such that the most common practice. We know from the early days of our schooling that iron is there. We know that iron can have two plus, or iron can have three plus so one we consider as a common name as the ferrous ion. Another is known as the ferric ion, so any of its ground states. That means whether it is present in the ferrous state or ferric state. We can have incompletely filled d level or the d shell, or the d orbitals, which will typically define whether this Fe 2+ or Fe 3+, both can be considered as the corresponding transition element derive derived ions. So, these are all transitions.
In the latter part of this chapter, we will first find what are those f block elements so before that we will just consider what are these d elements and what are the transition metal ions. By definition, a transition metal is an element whose atom has partially filled d sub shell. So, the occupancy of these d sub shells is important and can give rise to cations with an incomplete d sub shell. If it can give rise to some cations, which have in corporately filled d sub shell, then that metal or that element we consider as the d block element. So, you have the incompletely filled d orbitals. If we have the d shell or the d orbitals, then we will find that these are all incompletely filled and where this incompletely filled d, orbitals, or d shell is in its ground state or in any one of its oxidation states, which is therefore important that the ground state configuration should give us an importantly filled d shell or any of its oxidation states, so when we consider their possibility of the oxidation states, whether it has a one permanent or one very easily accessible, oxidation state or it can have the different oxidation state. Whatever oxidation state, you can have, whether you have an incompletely filled d shell or not, that will typically define whether you are talking about the corresponding element, which is a transition element such that the most common practice. We know from the early days of our schooling that iron is there. We know that iron can have two plus, or iron can have three plus so one we consider as a common name as the ferrous ion. Another is known as the ferric ion, so any of its ground states. That means whether it is present in the ferrous state or ferric state. We can have incompletely filled d level or the d shell, or the d orbitals, which will typically define whether this Fe 2+ or Fe 3+, both can be considered as the corresponding transition element derive derived ions. So, these are all transitions.
Ions or transition element ions, which can be derived from iron, which is Fe 0. So, in a similar way, we give the definition for f block, and in these two cases we just considered that, since the group will start now, we follow that the transition elements are following from periodic table after calcium. Similarly, here these are starting from lanthanum and actinium, so where the position of this lanthanum and the position of the actinium we should know and based on that, we basically consider that, following that, so once the lanthanum we reach, then the following electron configuration or the occupancy in the shell, which is whether d or f is different, and this occupancy will consider as again some type of transition metals, but this type, the occupancy is not d, but it could be occupancy of the f shell, which will basically give us something where we can have a group of elements or group of metal ions, which we will consider as inner transition elements because, after d, we just get again not an ultimate shell. But it is a penultimate shell below this 3d level is the inner transition, metals or inner transition, metal ions. Initially, if we just simply consider a part of the whole periodic table which belongs to the d block elements and those d block elements are important to understand. That means, on the left-hand side we have till calcium, which has the atomic number of 20, and, on the right-hand side we have the p block elements so in between we have the period three.
So, we have period one, period two, and period three. After we reach period 3, then only the possibility of coming to the corresponding d shell is there. After calcium, the first element will be scandium, so then we have the scandium, titanium, vanadium, chromium, manganese iron, cobalt, nickel, copper, and zinc. Already we have defined this. So, we are excluding these. That means zinc, cadmium, and mercury from this list because these are group 12 elements. That means the incompletely filled d shell, which cannot be applied to zinc in its ground state, or zinc in its typically available or most commonly available oxidation state, which is zinc 2 plus. So, we will just get for period 4, which is scandium to copper, and what are hose electronic configurations that will follow that these are occupying 3d levels, so these are also 3d elements or 3d block elements, starting from scandium to copper. Similarly, if we go to the next period, which is period 5, we get atrium to zirconium niobium to silver and cadmium, and similarly, period 6 will give you something where we have. You see that 57 to 71, these are. These are the f block elements and, after that, f block elements then only we will get the electron occupancy or electron filling to the d level, which is hafnium, then tantalum then tungsten to gold. These three mostly are commonly encountered. So, this particular group means from scandium to gold 79.
We see that if we just are at the group level also, the group similarity will also be there. So, all these we considered as the triad because these are not naturally occurring elements for period seven. Only some synthetically prepared elements have been accumulated over there and day by day. We are just filling up all these levels already. We have filled up all these levels till this 111 atomic number, but these three periods particularly period four period five, and period six are important to study these things, and we know that one. That means the clubbing of all these elements we put together. That means the three d elements or 3d block elements or d block elements for scandium to copper. We feel that their properties are changing as we move from scandium to titanium to vanadium, to nickel to copper. Similarly, as we move from this period, 4 to period 5 to period 6, what is changing? We are just changing from 3d to 4d to 5d elements so down the group. That means group four elements: group, five elements; group, six elements and group; seven elements and group eight elements so down the group how the properties of all these groups can change, because the ultimate electronic configuration will be same, such as that of our nickel, which is group 10 element and of 3d and of 4d it will be palladium and for 5d it will be platinum.
So, if we just consider something that means initially, you have we do not know much because we are not very much concerned about the corresponding chemistry of the metallic part because its relation to metallurgy and the metallic part or the alloy formation. But if we take out those two electrons that means two s: electrons, on the left-hand side, so s electrons will be lost first. So, we are remaining with the d electrons for its cationic form, which is in 2 plus. If we have in 2 plus from here, similarly, if we can have palladium 2 plus or if we can platinum, 2 plus in all these cases, we see that the corresponding configuration in terms of the occupancy in the d level will be 3d. Some number then 4d, some numbers and then 5 d sub number. Similarly, these properties, that means from iron, ruthenium, and osmium, but interesting thing is that, as we move down from iron to ruthenium to osmium and the size of the diesel or size of the d, orbitals are increasing enormously, and the corresponding properties and the reactivity patterns are also changing so the next thing. What will be seeing that how we can consider what should be the electronic configuration of say, scandium, 21 or say platinum 78. We should have some good ideas how quickly we can write that it has the ultimate electronic configuration for scandium.
Scandium 0 is 4s2 3d1. That means the first electron is entering in the level which is 3d, and that means that we have the unoccupied 3d level. So, scandium falls under that category of transition element, so titanium will be in the equivalent way. That means the 4 s2 3d2. So, what we are getting to start from group 3 to group 11. We are getting d1, d2, d3, d4, d5, d6, d7, d8 and d9 system. So, another way of classifying or placing all these in the periodic table is important such that we quickly consider that in a particular oxidation state, we can have electronic configuration, which is also known by its positioning in the corresponding group. So, what we see is that, in the long form of the periodic table, that means the color, which will be telling us that the pink color will be telling us that these are the transition metals so scandium to corresponding gold.
 So, this group, this group, and the left-hand side. We have the corresponding s, block elements and the right-hand side. We have the p block elements in this side and then the group, which is inert also we know, and as we move from here, that means after lanthanum, will be getting the corresponding occupancy like those 10 electrons in this particular group. Similarly, we have 14 electrons in the f level, so after lanthanum we get the series from here to here. That means cerium to lutecium. These are known as lanthanides. Similarly, after actinium, whatever element, that means the 14 elements will be posing there due to the occupancy of the corresponding 5f level are known as correspondingly actinides. So, these two groups will be coming over here before that.
We should finish our discussion in relation to the corresponding transition elements and mostly, we always concerned about the particular part, which is the first transition series, because we know very much because these are mostly commonly available on the earth crust, because as minerals and ores, the corresponding abundances are more even they have present in the biological form in the biological system, even in our body, because iron, we all know iron - is present in our body also and a particular process like the process, we call is a mineralization process that mineralization process is responsible For storing iron on the earth crust, similarly other process which we can consider in a similar way, that is the bio mineralization process and that bio mineralization process can be considered for storing iron in our body, also for the synthesis of hemoglobin and myoglobin like things.
So, this group, this group, and the left-hand side. We have the corresponding s, block elements and the right-hand side. We have the p block elements in this side and then the group, which is inert also we know, and as we move from here, that means after lanthanum, will be getting the corresponding occupancy like those 10 electrons in this particular group. Similarly, we have 14 electrons in the f level, so after lanthanum we get the series from here to here. That means cerium to lutecium. These are known as lanthanides. Similarly, after actinium, whatever element, that means the 14 elements will be posing there due to the occupancy of the corresponding 5f level are known as correspondingly actinides. So, these two groups will be coming over here before that.
We should finish our discussion in relation to the corresponding transition elements and mostly, we always concerned about the particular part, which is the first transition series, because we know very much because these are mostly commonly available on the earth crust, because as minerals and ores, the corresponding abundances are more even they have present in the biological form in the biological system, even in our body, because iron, we all know iron - is present in our body also and a particular process like the process, we call is a mineralization process that mineralization process is responsible For storing iron on the earth crust, similarly other process which we can consider in a similar way, that is the bio mineralization process and that bio mineralization process can be considered for storing iron in our body, also for the synthesis of hemoglobin and myoglobin like things.
So, these elements are so important that we should know very much because they have different interesting properties related to the transition elements. So, the definition is that for pink element, that we have partly filled d levels and for these two groups for lanthanides and actinides, we have the partly filled f shells. If we just consider that what about the period four transition metals so will be just knowing now that what are those metals, because we quickly will see that a particular type of metals. What we will see is that we can have these corresponding properties of these metals, particularly how we can store them, and just now I am giving some examples that iron we know that iron in a metallic form. We know that iron nail, we know iron nail or iron seed. We know the use of iron. We all know very much. Similarly, if we get something that the corresponding ions, Fe2+ and Fe3+, and if I now say that any of them is also present in our blood as hemoglobin and myoglobin. So, this thing will concern the corresponding transition metal ions. These are not metals, so the corresponding property of these things is that we have the corresponding property of these and how this iron will look like. Some of us have some good information about what an iron nail will look like. Iron seed will look like, but what are these particular things will be in solution so this whether this will be soluble in water, medium or any other medium and how they will also look like, and similarly some of these elements can also be useful for the alloy formation, so before going into that particular detail because iron, what we know that iron is also present from the ore and minerals, because this will all be present on the earth crust and if they are present, as some oxide and in all of our redox classes, then the previous classes, we have identified that how we can recover iron elemental iron or the metallic iron from all these odds. So, this is a typical process which the environment does for us. The earth is doing for us, and we are storing that one and when we recover so recovery process is typically the corresponding metallurgical process. So, this is the corresponding metallurgy we can have and that gives rise to iron 0, but how is this iron?
Iron will look like suppose if you are given with some iron powder, because it has some important property for this iron as a dust particle type of thing, so how this iron powder will look like so only some examples for this period, four transition metal. The first one is a typical example for scandium. This is the metallic scandium, so metallic scandium is there, which is the corresponding group, the element, and if we put that on a petri dish, the metallic form of scandium will look like of this type. Similarly, the titanium these are granules, so if we have the corresponding ore from the earth crust so or for the titanium also, we know that titanium dioxide tio2 is the typical ore for that, so titanium dioxide is there and from there we just have to go for the corresponding reduction, so the mechanism is there that how to get titanium from tio2, so in the metallic form, if we produce that thing so in the corresponding granules are forming and the titanium. So, with that thing will also give us the properties. What we have just now discussed is that it has luster, it has strength and all these, so the corresponding metallic properties for all these things will be there. So, we get that for the corresponding vanadium, also vanadium, we just once we move to vanadium.
Vanadium will also give us something where the color of these things is changing. So, if I put something that means how nicely we look at all these things, that means from the nature of all these things, particularly the color of all these and the metallic cluster of all these things, we can identify it immediately, whether it is scandium. This is titanium, and that is vanadium, so these all have different, so the nature of these units that means the corresponding granules the nature of these granules. These are not typical powder, because some other process we have to go for getting the corresponding powder. Similarly, chromium, you see the chromium, also look like a chromium powder, so this a chromium powder we can have. This is a powderier form. This has less metallic cluster type of character, so it is forming a typical powder type of thing, than manganese, you all know the most typical. Corresponding ore is the pyrolusite which is manganese dioxide, which is plentiful in nature. India is also very much rich in having manganese dioxide or pyrolysis, so, the mining process gives us the mining for manganese. We take out that ore and the industry. The metallurgical industry will give rise to the corresponding manganese. So, if we consider that in some cases, we get that thing that where we can have that manganese, typically in our hand, so manganese will be for the manganese metallic state. So, we can use this metallic state sometimes because most of these are as metal. They can also react nicely with the acids, so the oxidation process, because we all know now that they can liberate hydrogen from the acid. Direct reaction of all these can liberate hydrogen and the metal will go to the corresponding ions like that iron when it reacts with hydrochloric acid iron powder.
So, this powder from the petri dish is what we can take. If we react with hydrochloric acid, the corresponding salt, what will be getting is the ferric chloride and hydrogen evolution can take place, so the corresponding iron powder, which can also be identified from the ores like hematite and magnetite cobalt, is also very much like that of our vanadium case, so it has also typical luster. So is a typical globule having a sine appearance, so cyan appearance on the surface of this will tell you that this is a covalent thing. Then nickel is also of different nature. That means when we go for the corresponding crystallization from a molten state, because all these at elevated temperature we are getting as in the molten state, and when we go down to room temperature, they crystallize it out in a typical form. So, the metallic nickel will be separated out from in the fashion. Similarly, this is copper, so copper is also the last pieces which we will get base already. We are having four plus four plus eight plus nine elements we just reached there. Then we can have zinc.
 In zinc we have the 3d ten arrangement because the zinc granules powders and all these are important to understand the positioning of this particular as required. It is a transition element or not, but the zinc will not be a transition element because in the elemental state or in the metallic state it has the corresponding electronic configuration of 4s two 3d ten. So, if we just take out those two electrons, the electrons will go from the 4s level. The 4s two electron will give you a 4s zero electronic configuration leaving behind with 3d, ten electronic configurations so that 3d 10 electronic configuration will give you a filled 3d level. So, zinc will not be considered as a transition element. So already we have discussed how we can jot down these physical properties, so, as inferred by the name. The transition metals are metals and thus conductors of electricity, so whatever the species, what we are just now have seen as the corresponding metals, because in some of our next classes we will be discussing about the corresponding formation of the transition metals. If we have the corresponding metal, which just now we have seen as the Fe 0, so it has all metallic property inherent to it. But when we move from there to say, Fe 2+ or Fe 3+ this is the typical electron transfer process.
In zinc we have the 3d ten arrangement because the zinc granules powders and all these are important to understand the positioning of this particular as required. It is a transition element or not, but the zinc will not be a transition element because in the elemental state or in the metallic state it has the corresponding electronic configuration of 4s two 3d ten. So, if we just take out those two electrons, the electrons will go from the 4s level. The 4s two electron will give you a 4s zero electronic configuration leaving behind with 3d, ten electronic configurations so that 3d 10 electronic configuration will give you a filled 3d level. So, zinc will not be considered as a transition element. So already we have discussed how we can jot down these physical properties, so, as inferred by the name. The transition metals are metals and thus conductors of electricity, so whatever the species, what we are just now have seen as the corresponding metals, because in some of our next classes we will be discussing about the corresponding formation of the transition metals. If we have the corresponding metal, which just now we have seen as the Fe 0, so it has all metallic property inherent to it. But when we move from there to say, Fe 2+ or Fe 3+ this is the typical electron transfer process.
We all know, and this is the oxidation process, but whatever things will be produced in solution in water, and these will be present in aqua solution. These two are in aqua solution, so these ions are in solution. These are, we can consider them as transition metal ions, so whatever we have such as this present in blood, if they are present in blood in these two forms. That means either iron two or iron three or any other biological system. So those are considered transition metal ions. We should always be very particular that you have ions, so these are forming with the corresponding ions, not the corresponding metals. So, if we just consider that these metals and thus, they have the good conductors of electricity, so the iron wires and all these things we can have - we know aluminum wires, like that of we have iron wires, so that, then we have the good conductor of electricity, we are using electrical wires, the copper wires and they are highly dense.
So, there was a high density and high melting points and boiling points also, so if we consider that corresponding thing that what we get, the corresponding properties is due to the progressive filling of the d shell, but the filling of these levels will give you the corresponding metallic character of these, so the metals when we talk their properties are due to the corresponding zero form. That means that iron, zero or nickel zero have the typical metallic bonding. So, we will not consider all these things in this class, but we should have a little bit of an idea about what is called this metallic bonding. So, just now we have seen that in case of 4s element and the 4p elements we have the ionic bond, typical ionic bond, and the typical covalent bond and in between. We can have four 3d elements in the metallic state. They can have metallic bonding and in the metallic bonding case, also when we will consider the typical bonding for the corresponding metal ions, they are also will find some interesting thing for their participation in the corresponding bonding when they participate as the corresponding transition metal ions. But what about in the free form? That means in the zero form in the metallic form? They also participate for the corresponding delocalization of the d electrons, and that is why they increase. They are cohesion due to the large number of these electrons, because we know that the capacity of the electrons when it is filling in zinc when it is filling completely in cadmium, when it is filling completely in mercury, they have 10 electrons altogether. So, as we have discussed, what is the typical indication that we are not getting as this one for the mercury, which is a typically a different thing, which is the field d level?
So, all these not only mercury but starting from zinc, which is 3d. Zinc is filled as 4 s2, 3 d10, then cadmium, 5 s2, 4 d10 and then mercury 5 s2, 6 d10. These all will have lower melting points. Boiling Point is also less because they have full d sub shells and they do not participate much in delocalization and sharing of the electrons and they do not have very good dd bonding in relation to increase the corresponding metallic bonding, so the conduction band they form, but the corresponding dd bonding will not participate much in forming the corresponding character and, as a result, the highest one.
 That means the mercury will have a very low melting point of -38.83 °C or -37.89 °F is a liquid at room temperature. So, from that one that metallic property is not there, but it has other properties, though it is in liquid, so other metallic property will be there, but it is not a transition metallic property we just expect from there. The first 3d series we just now take out, partly because we will be talking about their properties, because what about these d block series? This d block series will be there if we just simply talk in terms of the corresponding appearance of the corresponding oxidation states, because just now we have seen from scandium to zinc how they look like now. If we take out the corresponding reactivity pattern of these. The one of the chemical reactivity, physical reactivity, we all know that how they form alloy, what is their metallic cluster, whether they are conductor all these, but what about their corresponding ionization? Ionization is their corresponding reactivity pattern with the acid whether your acid is oxidizing or not.
That means the mercury will have a very low melting point of -38.83 °C or -37.89 °F is a liquid at room temperature. So, from that one that metallic property is not there, but it has other properties, though it is in liquid, so other metallic property will be there, but it is not a transition metallic property we just expect from there. The first 3d series we just now take out, partly because we will be talking about their properties, because what about these d block series? This d block series will be there if we just simply talk in terms of the corresponding appearance of the corresponding oxidation states, because just now we have seen from scandium to zinc how they look like now. If we take out the corresponding reactivity pattern of these. The one of the chemical reactivity, physical reactivity, we all know that how they form alloy, what is their metallic cluster, whether they are conductor all these, but what about their corresponding ionization? Ionization is their corresponding reactivity pattern with the acid whether your acid is oxidizing or not.
That means the reaction with hydrochloric acid, the reaction with oxidizing acids like nitric, acid, or sulfuric acid, so that will give rise immediately, whether we are able to get the corresponding salt formation, that we have seen the zinc, the metallic zinc, or the zinc rod. In our previous redox chemistry classes, we have seen that zinc rod can lead to something where the evolution of hydrogen can take place and we can give rise to the corresponding metallic salt, starting from zinc oxide or the zinc itself. So, this thing is that now we can just separate it out quickly from group 3 to group 11, including group 12, because we reach the 3d 10. So, the atomic number, the elements, and the configurations so configurations we always we can have some good idea. So, separating out of these cadmium to zinc and what about this possibility is also there. That means we once we know that zinc then copper, then nickel, which is a 3d 10 element. Then we have the 4d, then 5d, so zinc, copper and then nickel. When we reach down to nickel, then downwards, we have the palladium, and we have the platinum.
Similarly, when we have iron 3 d6 4 s2. If we go what we are just now seeing that if we know the configuration, that means the positioning in the group, the corresponding atomic number also so this we can find out is not that you have to memorize all these things, but you should know that this atomic number once it is 26 by filling up the electrons from left to right from 21 to 30, where your position of the iron and its electronic configuration? It is 3 d6, 4 s2, so this is iron, which is in the zero state.
 So, when it is losing two electrons, it is losing three electrons so straight away. We will not consider electron occupancy at this level. We will write, that it is a 3d6 ion, so ferrous is a 3d6 ion and this particular case what we will see that we have the most two common oxidation states so for iron here we will just simply write as we know that also for our Blood in our body that either you have iron, two plus or iron, three plus, or something which is in between or something related to, its corresponding reduced form of the ferry.
Fe3+ the most common oxidation states, which is very important that how facile they are.
So, when it is losing two electrons, it is losing three electrons so straight away. We will not consider electron occupancy at this level. We will write, that it is a 3d6 ion, so ferrous is a 3d6 ion and this particular case what we will see that we have the most two common oxidation states so for iron here we will just simply write as we know that also for our Blood in our body that either you have iron, two plus or iron, three plus, or something which is in between or something related to, its corresponding reduced form of the ferry.
Fe3+ the most common oxidation states, which is very important that how facile they are.
That means the formation of these by simply reacting with, say, dilute hydrochloric acid, cold and dilute hydrochloric acid, which is aquas. So, the reactivity of the iron powder, how we have seen that what is iron powder, so the activity of these iron powder will just lead to the evolution of hydrogen, so hydrogen evolution can take place and the corresponding ions will remain as the chlorides are there. So, we will have the corresponding thing as the ferrous chloride and if it is oxidizing, because these the redox potential, the redox coupled between these two are less than e zero value for these two are also less, which is 0.7 volt. So, if oxygen is there, oxygen is much more oxidizing, so it is in air. If we handle everything in aqueous solutions already, the water present for this aqua solution or the preparation of this hydrochloric acid, so o2 is there. In this aqua solution o2 is the oxidizing one. So o2 is oxidizing agent, so that will immediately oxidize this to Fe 3+. So, what about this electronic configuration so electron configuration for Fe3+. We have to take out one electron from this. 3 d6, so it will not be 3 d6. It will be 3 d5. For these two that means we have 3 d6 ion and 3 d5 ion, which are the most common oxidation states for 3-d levels. So, since we are talking about 3-d, so if we just consider the periodic table so in the periodic table we have iron, ruthenium, and osmium so, which is 3d 4d and 5d, and these are having giving rise to some electronic configurations. If we consider that both are giving, that means the trivalent state, is stable state for iron, ruthenium, and osmium. So, this is the electronic configuration, which is 3d5 for iron 3 plus.
So, without knowing much or without bothering much about what would be for ruthenium, ruthenium will be ruthenium 3+ this is iron 3+, so iron 3+ will be 3 d5, so ruthenium 3+ will also be 4 d5. Similarly, osmium can go for osmium 3+ which will be 5 d5. So, this is the advantage of knowing the periodicity of the elements, placing them in the periodic table and how quickly we can understand when we talk about the chemistry of these, because sometimes we can handle all these in solution having some test tubes, we can have some Test tubes test one test tube two and test tube three. In one case, we have ferric ion in solution, in another case ruthenium in the trivalent state, in other case the osmium in the trivalent state. So, the generalization of all these things is very important, and we know that most of these cases we are removing the electrons from the d level. That means this oxidation. The first one electron loss, one electron loss. The first one electron loss for getting ferric ion from ferrous ion is the removal of the electron from the d level. So, this is much more facile, but if we can have some arrangement and if we can have some stronger oxidizing agent, then we can find out whether we will be able to take out the electrons from their corresponding positioning. That means whether we can go beyond that means whether we can take out more electrons from these. That means we can take out one more electron from this level, giving three d four or three d: three, so those oxidation states we can get, and those oxidation states will be termed as unusual oxidation states or uncommon one so uncommon, oxidation states we can have. That means beyond this, so 2 and 3 can be there, so we can have 4 plus we can have 5 plus, or we can have 6 plus, but all together what we can have all together.
We can have 8 electrons 2 in s level and 6 in d level. So, if we remove all these electrons from s level and d level will be getting something which is eight plus. So, whether we will be getting that one is important to discuss whether it is possible for iron and whether it is possible for all other elements. So, what we see that getting all these oxidation states, that means plus two plus three plus four plus five and plus six, so these elements, these transition elements, therefore occur in variable oxidation states, so they occur in variable oxidation states, so one or the other. That means, as we move, that means the corresponding filling off the d level from scandium to iron. We are filling up stepwise one after another, one electron, two electron three electron, four electron, five electron and six electrons. Similarly, when we are talking in terms of the removal of electrons from that d level or d shell is the corresponding oxidation reaction, so solution, chemistry for all these metal ions is, will be mostly dominated by the presence of the corresponding oxidation states, and we should Have some good knowledge about the presence of all these oxidation states in our hand?
 So, we get this as the 3d elements or d block series in the third level. Similarly, we get the next one, which is the second d block series, which is from atrium to cadmium and again, like that of our electronic configuration. The positioning of all these things. We see that in this case also, the progressive filling of the d shell is important, and we have in some cases we have the mostly from here that means d 1 to d 9, because this we just move for that. If we move this electron to the s level, which will be 5 h2 and which will be 3d 4d9, we get the progressive filling of the d shell, so we will get these, and since this one we are talking about so this one since the size Is increasing instead of writing as d6 s2 electronic configuration we can move, because these are close by energy wise. These levels are close by the d level and the s level, so we can move this electron to this particular shell. So that is the configuration is now 4d7 5s1. So that tells us something that whether we can have that removal of single one electron from the s level. So, removal of that one electron will give rise to a state where you can have ruthenium.
So, we get this as the 3d elements or d block series in the third level. Similarly, we get the next one, which is the second d block series, which is from atrium to cadmium and again, like that of our electronic configuration. The positioning of all these things. We see that in this case also, the progressive filling of the d shell is important, and we have in some cases we have the mostly from here that means d 1 to d 9, because this we just move for that. If we move this electron to the s level, which will be 5 h2 and which will be 3d 4d9, we get the progressive filling of the d shell, so we will get these, and since this one we are talking about so this one since the size Is increasing instead of writing as d6 s2 electronic configuration we can move, because these are close by energy wise. These levels are close by the d level and the s level, so we can move this electron to this particular shell. So that is the configuration is now 4d7 5s1. So that tells us something that whether we can have that removal of single one electron from the s level. So, removal of that one electron will give rise to a state where you can have ruthenium.
In one plus state, so in a particular condition, or in a situation that we will find afterwards that one type of compound, we call as the organometallic compounds where we can have some interaction of this metallic state in the zero. That means the powders can react with some of the species like that of our metallic state, which can interact with that thing. That means simple carbon monoxide, so the 3d container of this palladium is nickel. We all know that nickel can interact with carbon monoxide, giving rise to tetra carbon in nickel 0., so nickel will be 0 in that case is the organometallic compound and that organometallic compound will have electronic configuration. We just consider that electronic configuration in terms of nickel zero. Similarly, if we something that palladium zero in different organic chemistry reactions the palladium zero, the metallic state of palladium is important and the corresponding electronic configuration. If we push all these electrons to the d level because this has an extra stability, the stabilization, we all know that the huff field shell - that is why this particular shell - we are writing. Instead of five s, two four d: four, we write as four d. Five s one, so one electron will move from s level to d level, so it has some extra stability. That means the half field shell and the full field shell. So, the palladium in that case that this, the palladium in the zero state will have a fulfilled state and that fulfilled d level will have a 4 d 10 electronic configuration and is stable.
 Similarly, the other one. That means the 5-d block and the 5-d block will get the corresponding one from that, lutetium to length or this lanthanum. This lanthanum is seventy-one. This not mutation is lengthening. It would be from hafnium to this gold, so there we are also having just simply the same type of electronic configuration and same number of electrons in the d level and the s level. But the thing is that now we can have the corresponding occupancy of these levels and it is changing from one level to the other. We are talking about something related to 3d level, 4d level and 5d level, so the corresponding occupancy of the corresponding period. That means the period which we are talking about for 3d, 4d and 5d. So, in this particular case, these are the 5d element, so the cationic state, just now what we have discussed that osmium like iron, so after iron we have ruthenium and then we have osmium. So, osmium is the congener of the iron group. So, in that particular case, we should not forget the group number similar fashion. We should know the atomic number also nicely, and the osmium and osmium are in plus two oxidation state, like that of our iron will be 5 d6 electronic configuration.
Similarly, the other one. That means the 5-d block and the 5-d block will get the corresponding one from that, lutetium to length or this lanthanum. This lanthanum is seventy-one. This not mutation is lengthening. It would be from hafnium to this gold, so there we are also having just simply the same type of electronic configuration and same number of electrons in the d level and the s level. But the thing is that now we can have the corresponding occupancy of these levels and it is changing from one level to the other. We are talking about something related to 3d level, 4d level and 5d level, so the corresponding occupancy of the corresponding period. That means the period which we are talking about for 3d, 4d and 5d. So, in this particular case, these are the 5d element, so the cationic state, just now what we have discussed that osmium like iron, so after iron we have ruthenium and then we have osmium. So, osmium is the congener of the iron group. So, in that particular case, we should not forget the group number similar fashion. We should know the atomic number also nicely, and the osmium and osmium are in plus two oxidation state, like that of our iron will be 5 d6 electronic configuration.
 So, all these things and the bonding in all these cases, what we see is related to the corresponding occupancy of all these d levels and we will just be able to consider the different thing. That means the 3d 4d and 5d elements. So, these 3d 4d and 5d elements, so we have so if we just consider these 3d 4d and 5d elements in their elemental state. That means M is in zero state, so physical properties are also changing. When we talk about bond strength, bond strength will also be changing, and this bond strength trend is there.
So, all these things and the bonding in all these cases, what we see is related to the corresponding occupancy of all these d levels and we will just be able to consider the different thing. That means the 3d 4d and 5d elements. So, these 3d 4d and 5d elements, so we have so if we just consider these 3d 4d and 5d elements in their elemental state. That means M is in zero state, so physical properties are also changing. When we talk about bond strength, bond strength will also be changing, and this bond strength trend is there.
So, as we go for bigger and bigger d level or d shell, the bond strength, strength will be changing and which is a different one, which is a reverse one for this, so this strain, this trend is reverse to that. Normally we found for main group elements that means the s block and p block elements. So, what we find for main group element is different for these transition elements. That means, if we consider that what about tungsten then, which is in the chromium group, so we have chromium, molybdenum, and tungsten, so chromium, molybdenum, and tungsten. We have, and in this particular case chromium. We know that this has six unpaired electrons. Similarly, molybdenum will also have six unpaired electrons, so we have five d4 and six s two. So, all these six electrons, if we consider the corresponding property as its zero state, that means tungsten in the metallic state, so the tungsten in the metallic state have the six electron and these six unpaired electrons.
They participate strongly in metallic bonding. So, we have a large number of electrons which are not possible to get for s level or the p level elements, so a large number of electrons are available for these. So as a result, they can have also very high electronegativity. So, tungsten will therefore have very high electronegativity, and this particular information is also important from our early school days. We know that they can be utilized, so they have a very high melting point and high boiling point. So, tungsten, metallic tungsten will have very high melting and high boiling point and, as a result, they can be utilized for making the bulb filament. So, the bulb filaments for incandescent lamps, we use tungsten as the corresponding material for the making of these bulb filaments.
So, we can have these.
 That means we just corresponding unpaired electrons, so we change the corresponding a melting point, so we just can see also what about the corresponding melting point trends also, so, as we move from scandium to titanium to zinc, so we find that the typical melting point in degree, centigrade will also be changing and which are above 100. So mostly, it is above 1000. So not sorry about thousands, so above 1000 °C and in some cases, they can go up to 3000 degrees centigrade. So, one value is 1539 °C for scandium, so it is increasing for titanium. It is increasing for vanadium as well as chromium, but in case of zinc it is less since those number of electrons which are there but is in the field shell.
That means we just corresponding unpaired electrons, so we change the corresponding a melting point, so we just can see also what about the corresponding melting point trends also, so, as we move from scandium to titanium to zinc, so we find that the typical melting point in degree, centigrade will also be changing and which are above 100. So mostly, it is above 1000. So not sorry about thousands, so above 1000 °C and in some cases, they can go up to 3000 degrees centigrade. So, one value is 1539 °C for scandium, so it is increasing for titanium. It is increasing for vanadium as well as chromium, but in case of zinc it is less since those number of electrons which are there but is in the field shell.
It is not available for that kind of metallic bonding, so a minimum will be finding over here where the levels are filled. So, the melting point minima will be finding over here and melting point. Maxima will be here for the transition metal ion. So thus, we see that the number of electrons in the elemental state will not forget that all are in the elemental state. That means the scandium as the metallic scandium titanium as the metallic scandium. They have a high melting point high boiling point, and some of these uses related to the metallic state, so the next day will be just considering how we get for the corresponding electron transfer reaction for the oxidation. That means the availability of the different oxidation steps. Ok, thank you very much.










