Chemistry Class 12 Unit 04 Chapter 01 Chemical Kinetics L 1 16 N5Vq9Bmob U En Punc Para Txt
Welcome students to today’s lecture, my name is Pramit Chowdhury, and I am a faculty in the department of chemistry at Indian institute of technology, IIT Delhi. So, the topic we are going to discuss in today’s lecture and in the fore coming lectures is chemical kinetics as is written on this piece of paper. Now before we go on to the details of chemical kinetics let’s try to understand the importance of chemical kinetics. So, when we say chemical kinetics, both of these words have very significant implications. So, for example, when we are talking about chemical kinetics, that means we are talking about processes related to chemistry or chemical processes.
For example, one kind of change might be a reaction of A + B → C, where A and B are the products and sorry are the reactants and C is the product also now this is a this is the case where there are two reactants, which are combining to give a product right. Now there can be another case where I have just a transformation: for example, a state A right see in one phase 1 to the same A but in phase 2. So, in the second case, what has happened? If I had a phase transformation. For example, say I am going from you know, ice to liquid water, or I am going from liquid water to water vapor that’s what the second one is talking about. Now, you know like this there are many examples in chemistry right, so if you consider these two, what they are representing is they are representing change or changes happening in whatever you are dealing with, or whatever is in front of you or whatever you are working on.
 Then what about kinetics? Kinetics is going to tell you how fast or how quickly this change happens right. So, if you write this, then what kinetics refers to is how fast or how quickly the particular process is going on. Then, essentially, what we are saying is we are saying what is the rate of that process now, if you think about this, why do we need chemical kinetics see all almost all of you have been taught or you yourself have studied about thermodynamics, the importance of thermodynamics in chemistry. Now, if you talk about thermodynamics in chemistry, what does thermodynamics tell you? Is there any need for us to go for chemical kinetics? Can we not get it from thermodynamics itself? So, let us talk about thermodynamics a little bit so that we understand the necessity of this topic or the significance of this topic to chemistry.
So, in thermodynamics, when we are talking about thermodynamics, remember if we write and think about thermodynamics, then this is what we are focusing on. It is about the initial state at the initial state right of your reaction or whatever, and we refer to it as I, then you have the final state which you refer to as f. So, thermodynamics mainly deals with these two states. Only the initial state, when you start a reaction and the final state. What is the final state? The final state is when you have when you have reached a chemical equilibrium, and that is why it is also referred to as chemical thermodynamics but see what is happening is yes, you are talking about the initial state. You are talking about the final state good, but what is happening in between you are not being able to focus too much on, for example, you know think about a certain process say ice going to water liquid right now.
What will thermodynamics tell you? Thermodynamics tells you that if I have to make this transformation from ice to water right, I will need to supply heat so that this transformation can be brought about, which tells me that the process, thereby this process, is endothermic. Similarly, if I go from liquid water to water vapor right, which is the gaseous state again, what you are doing is you are transforming the molecules, the same water molecules from the liquid state to a gaseous state. Again, you are supplying energy, so this process is also endothermic, and thermodynamics tells you that that you have to supply heat so that this process or this transformation is being brought about along with this. So, this is only a part of it. Along with this, there are some other very common thermodynamic parameters that you can obtain or get from these sorts of reactions.
Then what about kinetics? Kinetics is going to tell you how fast or how quickly this change happens right. So, if you write this, then what kinetics refers to is how fast or how quickly the particular process is going on. Then, essentially, what we are saying is we are saying what is the rate of that process now, if you think about this, why do we need chemical kinetics see all almost all of you have been taught or you yourself have studied about thermodynamics, the importance of thermodynamics in chemistry. Now, if you talk about thermodynamics in chemistry, what does thermodynamics tell you? Is there any need for us to go for chemical kinetics? Can we not get it from thermodynamics itself? So, let us talk about thermodynamics a little bit so that we understand the necessity of this topic or the significance of this topic to chemistry.
So, in thermodynamics, when we are talking about thermodynamics, remember if we write and think about thermodynamics, then this is what we are focusing on. It is about the initial state at the initial state right of your reaction or whatever, and we refer to it as I, then you have the final state which you refer to as f. So, thermodynamics mainly deals with these two states. Only the initial state, when you start a reaction and the final state. What is the final state? The final state is when you have when you have reached a chemical equilibrium, and that is why it is also referred to as chemical thermodynamics but see what is happening is yes, you are talking about the initial state. You are talking about the final state good, but what is happening in between you are not being able to focus too much on, for example, you know think about a certain process say ice going to water liquid right now.
What will thermodynamics tell you? Thermodynamics tells you that if I have to make this transformation from ice to water right, I will need to supply heat so that this transformation can be brought about, which tells me that the process, thereby this process, is endothermic. Similarly, if I go from liquid water to water vapor right, which is the gaseous state again, what you are doing is you are transforming the molecules, the same water molecules from the liquid state to a gaseous state. Again, you are supplying energy, so this process is also endothermic, and thermodynamics tells you that that you have to supply heat so that this process or this transformation is being brought about along with this. So, this is only a part of it. Along with this, there are some other very common thermodynamic parameters that you can obtain or get from these sorts of reactions.
 So, common things you ask for are - or you know, parameters you ask for like the free energy change of the reaction or process right. So, this free energy change is often referred to as ΔG, and we know so. I am not going to write that you know that ΔG is negative. That means the process is spontaneous if the ΔG is positive, the process is a non-spontaneous process. Also, you can talk about entropy changes which are given by ΔS. So, so far, so good right, you have the initial state, you have the final state and because you are talking about ΔG, ΔS or ΔH right, whether it’s ΔH is endothermic so, that means you are supplying heat or exothermic that means heat is getting released, so ΔH is negative right. So, for an example, if you talk about dilution of concentrated sulfuric acid (H2SO4), ok, let us talk about this very briefly. So, you have very concentrated sulfuric acid (H2SO4). What you do is you take some sulfuric acid (H2SO4) from the chemical reagent bottle and dilute it so I will give you some measures of the heat that is released. This is a highly exothermic process.
So, for example, suppose you are having say this is H2SO4 in milliliters, considered sulfuric acid (H2SO4). Remember you have taken it directly from the reagent bottle, concentrated reagent bottle. Then you have H2O in milliliters. Ok, you are mixing these, so how are you mixing? So, suppose the volume of H2O is 100 ml and the volume of H2SO4 you are adding to this 100 ml of water is 10 ml. So, then the ΔH, that means enthalpy change of this reaction in kilojoules is - 11 kilojoules right and then the corresponding change in temperature is 25 degrees Celsius. What does this mean? What it means is when you are taking 100 ml of water say in a beaker or in a suitable container. You are adding 10 ml of concentrated sulfuric acid (H2SO4) then this amount of heat is released, and the temperature increases to 25. So, that is why the container feels so warm. So, this is an exothermic process, exothermic being referred to or being signified by the presence of this negative sign. Ok now let’s increase the volume of sulfuric acid (H2SO4), so added say: if you go to 30 ml of sulfuric acid (H2SO4), again added to the same amount of water, then the heat released is about -30 kilojoules and the temperature change is about 70 degrees Celsius.
So, you can immediately understand, while in one case well in one case, say, for example, the phase transformation of ice to water, liquid water and liquid water to water vapor. You had to supply heat so that they can make the next transformation or go to the next phase. In this case, when you are diluting sulfuric acid (H2SO4), concentrated sulfuric acid in water, you are having a huge amount of heat coming out or getting released, and that is why the container also feels very hot or warm depending upon the amount of temperature change you are having. Hence this process. Hence this process of dilution is referred to as an exothermic process. Ok, so then this again comes under the rearm of thermodynamics, because thermodynamics is telling you that ok, this is the energy that is coming out because of the dilution, or this is the energy of the supply so as to bring about the phase change for the previous endothermic processes, but you have to realize one thing now: if you ask the question: how long does it take for the phase change to happen? How long does it take for the reaction to happen for any reaction to happen? Thermodynamics does not give you an answer for that.
So, then we can write from thermodynamics from thermodynamics. We do not have any information about time. Ok, so if I can write it again, I can say that thermodynamics gives me no time information gives me no information about time, so the only way I can get information about the time that is, the rate at which this transformation or any transformation is taking place, is to resort to or take help of chemical kinetics. That’s why this topic in itself has such a significant place in the heart of chemistry or as a topic it is so very important to chemistry. Ok, now, when you talk about kinetics, as we just said, we are mainly interested in the time taken right. How slow, how fast? It’s one also question that is remembered when we are talking about thermodynamics. We said that calculations are involved when they include this ΔH or ΔG or ΔS. We say that this is the difference between the final state and the initial state. So, these are the only two states we are concerned about in thermodynamics all the time.
So, common things you ask for are - or you know, parameters you ask for like the free energy change of the reaction or process right. So, this free energy change is often referred to as ΔG, and we know so. I am not going to write that you know that ΔG is negative. That means the process is spontaneous if the ΔG is positive, the process is a non-spontaneous process. Also, you can talk about entropy changes which are given by ΔS. So, so far, so good right, you have the initial state, you have the final state and because you are talking about ΔG, ΔS or ΔH right, whether it’s ΔH is endothermic so, that means you are supplying heat or exothermic that means heat is getting released, so ΔH is negative right. So, for an example, if you talk about dilution of concentrated sulfuric acid (H2SO4), ok, let us talk about this very briefly. So, you have very concentrated sulfuric acid (H2SO4). What you do is you take some sulfuric acid (H2SO4) from the chemical reagent bottle and dilute it so I will give you some measures of the heat that is released. This is a highly exothermic process.
So, for example, suppose you are having say this is H2SO4 in milliliters, considered sulfuric acid (H2SO4). Remember you have taken it directly from the reagent bottle, concentrated reagent bottle. Then you have H2O in milliliters. Ok, you are mixing these, so how are you mixing? So, suppose the volume of H2O is 100 ml and the volume of H2SO4 you are adding to this 100 ml of water is 10 ml. So, then the ΔH, that means enthalpy change of this reaction in kilojoules is - 11 kilojoules right and then the corresponding change in temperature is 25 degrees Celsius. What does this mean? What it means is when you are taking 100 ml of water say in a beaker or in a suitable container. You are adding 10 ml of concentrated sulfuric acid (H2SO4) then this amount of heat is released, and the temperature increases to 25. So, that is why the container feels so warm. So, this is an exothermic process, exothermic being referred to or being signified by the presence of this negative sign. Ok now let’s increase the volume of sulfuric acid (H2SO4), so added say: if you go to 30 ml of sulfuric acid (H2SO4), again added to the same amount of water, then the heat released is about -30 kilojoules and the temperature change is about 70 degrees Celsius.
So, you can immediately understand, while in one case well in one case, say, for example, the phase transformation of ice to water, liquid water and liquid water to water vapor. You had to supply heat so that they can make the next transformation or go to the next phase. In this case, when you are diluting sulfuric acid (H2SO4), concentrated sulfuric acid in water, you are having a huge amount of heat coming out or getting released, and that is why the container also feels very hot or warm depending upon the amount of temperature change you are having. Hence this process. Hence this process of dilution is referred to as an exothermic process. Ok, so then this again comes under the rearm of thermodynamics, because thermodynamics is telling you that ok, this is the energy that is coming out because of the dilution, or this is the energy of the supply so as to bring about the phase change for the previous endothermic processes, but you have to realize one thing now: if you ask the question: how long does it take for the phase change to happen? How long does it take for the reaction to happen for any reaction to happen? Thermodynamics does not give you an answer for that.
So, then we can write from thermodynamics from thermodynamics. We do not have any information about time. Ok, so if I can write it again, I can say that thermodynamics gives me no time information gives me no information about time, so the only way I can get information about the time that is, the rate at which this transformation or any transformation is taking place, is to resort to or take help of chemical kinetics. That’s why this topic in itself has such a significant place in the heart of chemistry or as a topic it is so very important to chemistry. Ok, now, when you talk about kinetics, as we just said, we are mainly interested in the time taken right. How slow, how fast? It’s one also question that is remembered when we are talking about thermodynamics. We said that calculations are involved when they include this ΔH or ΔG or ΔS. We say that this is the difference between the final state and the initial state. So, these are the only two states we are concerned about in thermodynamics all the time.
 However, in case of kinetics, you start asking this question: if I have a process, so that means, if I have A → B - and if this is a process, then I start asking: how does this process happen? So, when you ask this question, which everybody should right, so the biggest question is how, what takes place for this change to happen, then it’s not only the time you are referring to well time is definite aspect, but what you also referring to is the mechanism, the mechanism at the molecular level that is, mechanism at the molecular level you need to know if I have to go from A to B, then what is taking place at the level of the molecules in that reaction system or in that container, so that this transformation or following which this transformation is happening, that is A → B. So, this also is addressed by chemical kinetics. This means you can immediately understand the significance. Hopefully, it is becoming a little more relevant in terms of discussion of chemical kinetics in chemistry, which is not only the rate. Yes, how fast? How slow, but also when this transformation is taking place on when this process is taking place, what steps might come in or what is the mechanism through which that particular process is happening? All these can be addressed through kinetics.
However, in case of kinetics, you start asking this question: if I have a process, so that means, if I have A → B - and if this is a process, then I start asking: how does this process happen? So, when you ask this question, which everybody should right, so the biggest question is how, what takes place for this change to happen, then it’s not only the time you are referring to well time is definite aspect, but what you also referring to is the mechanism, the mechanism at the molecular level that is, mechanism at the molecular level you need to know if I have to go from A to B, then what is taking place at the level of the molecules in that reaction system or in that container, so that this transformation or following which this transformation is happening, that is A → B. So, this also is addressed by chemical kinetics. This means you can immediately understand the significance. Hopefully, it is becoming a little more relevant in terms of discussion of chemical kinetics in chemistry, which is not only the rate. Yes, how fast? How slow, but also when this transformation is taking place on when this process is taking place, what steps might come in or what is the mechanism through which that particular process is happening? All these can be addressed through kinetics.
 Now, once you think about this, you start asking other questions right, so suppose you are thinking about the rate of the reaction of any particular reaction, so I say rate of reaction, then immediately the question that comes to your mind: Can I control the rate of the reaction? If I can, what are the factors? So, that means the first question that comes to your mind is: can I have control of the rate of the reaction? You say I say yes, then your next question is, great, if so, please tell me how I can control that means what are the factors that will control the reaction rate? Now, as we go through our lectures, we are going to spend time on this and discuss the different factors, but I am sure most of you already know and can already realize that some of the factors are very commonly used factors that can control the rate, so one would be concentration, then another one would be temperature so generally with increase in temperature the reaction rate increases and then there is something which has a very unique place in chemistry , catalyst. So, a catalyst is something which increases the rate of the reaction.
So, that means, if you have to control the rate of a reaction, suppose you see that the reaction went too fast. You are doing an experiment in a certain laboratory, so you are doing a practical experiment say in one of your practical classes and then you are following this transformation say from A to B and you certainly found out this reaction just went too fast for me to capture what was happening or to capture the rate, because it was just too fast. So, how can I decrease the rate one, I can play with the concentration two, I can play with the temperature and catalyst also has its own unique place, which we will discover later as we go through this course more and more ok.
Now kinetics is mainly referred to as a branch of physical chemistry, ok, but this is generally what you know kinetics is thought as being a branch of physical chemistry, but you know what, if you really think about kinetics, it is actually a unifying topic. So, kinetics I can see it is a unifying topic covering many branches, so it has relevance in biochemistry, it is applicable in biology right, now talk about mechanisms in organic and inorganic chemistry. The moment you talk about mechanisms is the very moment you also start talking about kinetics. Again, how fast, how slow do these things happen? Can I speed the reaction up by adding a catalyst? Can I speed the reaction up by changing the concentration of this? So, what it means is that kinetics, the importance of kinetics is not only in the branch of physical chemistry as it’s supposed to be, but it really is spread over all branches, and that is why the relevance of kinetics are the importance of kinetics and hence I think this is a very good starting point based on which we can build on this topic or this concept of chemical kinetics. But you know before I discuss the rate, equations and other aspects or features of chemical kinetics I would like to discuss some examples with you in daily life, where chemical reactions and that to kinetics become quite important.
So, as an example, first, I will discuss chemistry in cars. Now you must have seen cars flying on the roads right nowadays there are many cars on the roads and many different car companies like Honda, Hyundai. You know many different car companies Maruti now what happens is the way the cars run, so at different places in cities or on the highways you would see that there are petrol pumps where the tank needs to be filled with petrol. Now this petrol, which the car runs on this petrol or gasoline named as, is a mixture of hydrocarbons. Ok, it is a mixture of hydrocarbons. You can say this CxHy right, so the hydrocarbon I am referring to is the generic symbolism. Where I have x atoms of carbon and y atoms of hydrogen right, so if it is methane, so suppose, if it is methane CH4 then x is equal to 1, y is equal to 4. If it is ethane C2H6, then I have x equal to 2, y equal to 6 and so on.
Now what happens is when you turn a car on this petrol which had filled the tank with from the petrol pump is burnt, so this petrol during the running of the car, the petrol is burnt right now when the petrol is burnt. So, that means the hydrocarbons are getting burnt, if it is an ideal condition then this is typically what you would get. So, that means CxHy, would combine with say the oxygen of air to give you CO2 and H2O. So, this is what you expect under ideal conditions if the fuel I am taking, which is composed of these mixtures of hydrocarbons, is being burnt or the fuel is being burnt, then they are burning, full burning and ideal burning and why I am talking about ideal you will soon realize. So, the ideal burning should lead to the formation of carbon dioxide and water, which are not very harmful. However, what happened is, now this is an ideal case right? Now suppose there is incomplete fuel burning, so that means all fuel is not getting burnt. If all fuel is not getting burnt, then what can happen is I can have some unburned hydrocarbon still out there, not only that when you burn this, what happens is you give rise to a high temperature, that means the temperature rises when the temperature rises and also because of incomplete burning.
Now, once you think about this, you start asking other questions right, so suppose you are thinking about the rate of the reaction of any particular reaction, so I say rate of reaction, then immediately the question that comes to your mind: Can I control the rate of the reaction? If I can, what are the factors? So, that means the first question that comes to your mind is: can I have control of the rate of the reaction? You say I say yes, then your next question is, great, if so, please tell me how I can control that means what are the factors that will control the reaction rate? Now, as we go through our lectures, we are going to spend time on this and discuss the different factors, but I am sure most of you already know and can already realize that some of the factors are very commonly used factors that can control the rate, so one would be concentration, then another one would be temperature so generally with increase in temperature the reaction rate increases and then there is something which has a very unique place in chemistry , catalyst. So, a catalyst is something which increases the rate of the reaction.
So, that means, if you have to control the rate of a reaction, suppose you see that the reaction went too fast. You are doing an experiment in a certain laboratory, so you are doing a practical experiment say in one of your practical classes and then you are following this transformation say from A to B and you certainly found out this reaction just went too fast for me to capture what was happening or to capture the rate, because it was just too fast. So, how can I decrease the rate one, I can play with the concentration two, I can play with the temperature and catalyst also has its own unique place, which we will discover later as we go through this course more and more ok.
Now kinetics is mainly referred to as a branch of physical chemistry, ok, but this is generally what you know kinetics is thought as being a branch of physical chemistry, but you know what, if you really think about kinetics, it is actually a unifying topic. So, kinetics I can see it is a unifying topic covering many branches, so it has relevance in biochemistry, it is applicable in biology right, now talk about mechanisms in organic and inorganic chemistry. The moment you talk about mechanisms is the very moment you also start talking about kinetics. Again, how fast, how slow do these things happen? Can I speed the reaction up by adding a catalyst? Can I speed the reaction up by changing the concentration of this? So, what it means is that kinetics, the importance of kinetics is not only in the branch of physical chemistry as it’s supposed to be, but it really is spread over all branches, and that is why the relevance of kinetics are the importance of kinetics and hence I think this is a very good starting point based on which we can build on this topic or this concept of chemical kinetics. But you know before I discuss the rate, equations and other aspects or features of chemical kinetics I would like to discuss some examples with you in daily life, where chemical reactions and that to kinetics become quite important.
So, as an example, first, I will discuss chemistry in cars. Now you must have seen cars flying on the roads right nowadays there are many cars on the roads and many different car companies like Honda, Hyundai. You know many different car companies Maruti now what happens is the way the cars run, so at different places in cities or on the highways you would see that there are petrol pumps where the tank needs to be filled with petrol. Now this petrol, which the car runs on this petrol or gasoline named as, is a mixture of hydrocarbons. Ok, it is a mixture of hydrocarbons. You can say this CxHy right, so the hydrocarbon I am referring to is the generic symbolism. Where I have x atoms of carbon and y atoms of hydrogen right, so if it is methane, so suppose, if it is methane CH4 then x is equal to 1, y is equal to 4. If it is ethane C2H6, then I have x equal to 2, y equal to 6 and so on.
Now what happens is when you turn a car on this petrol which had filled the tank with from the petrol pump is burnt, so this petrol during the running of the car, the petrol is burnt right now when the petrol is burnt. So, that means the hydrocarbons are getting burnt, if it is an ideal condition then this is typically what you would get. So, that means CxHy, would combine with say the oxygen of air to give you CO2 and H2O. So, this is what you expect under ideal conditions if the fuel I am taking, which is composed of these mixtures of hydrocarbons, is being burnt or the fuel is being burnt, then they are burning, full burning and ideal burning and why I am talking about ideal you will soon realize. So, the ideal burning should lead to the formation of carbon dioxide and water, which are not very harmful. However, what happened is, now this is an ideal case right? Now suppose there is incomplete fuel burning, so that means all fuel is not getting burnt. If all fuel is not getting burnt, then what can happen is I can have some unburned hydrocarbon still out there, not only that when you burn this, what happens is you give rise to a high temperature, that means the temperature rises when the temperature rises and also because of incomplete burning.
 You can have other reactions happening. For example, this incomplete burning of CxHy can give rise to not carbon dioxide, but carbon monoxide as one of the gases is coming out. Then where are you getting the oxygen from? You are getting oxygen from the air is also has lots of nitrogen. So, what can also happen is that nitrogen can combine during burning to give rise to nitric oxides, NOx, so this NOx is typically composed of NO and NO2. So, this one, you know, is nitrogen dioxide (NO2), and this one is nitric oxide (NO), so see what has happened.
The ideal condition was this, that you have fuel it combines with the oxygen of the air, and it gives rise to carbon dioxide and water. This is the ideal condition good, but then under non ideal cases, this is typically what happens you know like when you read about ideal gas, non-ideal gas. See, ideal gas is an ideal condition. Mostly all gases are non-ideal in nature. Similarly, here incomplete fuel burning gives rise to some gases which we do not want which are poisonous for us. I will come to that very soon, but what are those gases? One is unburned hydrocarbon. Then you also have this unbound hydrocarbon, reacting with oxygen going to CO, which is again incomplete combustion. That means it does not go to CO2. You have nitrogen from the air now which can combine at this high temperature to give rise to oxides of nitrogen represented as NOx and under this NOx umbrella. We have NO, which is nitric oxide and NO2, which is nitrogen dioxide.
You can have other reactions happening. For example, this incomplete burning of CxHy can give rise to not carbon dioxide, but carbon monoxide as one of the gases is coming out. Then where are you getting the oxygen from? You are getting oxygen from the air is also has lots of nitrogen. So, what can also happen is that nitrogen can combine during burning to give rise to nitric oxides, NOx, so this NOx is typically composed of NO and NO2. So, this one, you know, is nitrogen dioxide (NO2), and this one is nitric oxide (NO), so see what has happened.
The ideal condition was this, that you have fuel it combines with the oxygen of the air, and it gives rise to carbon dioxide and water. This is the ideal condition good, but then under non ideal cases, this is typically what happens you know like when you read about ideal gas, non-ideal gas. See, ideal gas is an ideal condition. Mostly all gases are non-ideal in nature. Similarly, here incomplete fuel burning gives rise to some gases which we do not want which are poisonous for us. I will come to that very soon, but what are those gases? One is unburned hydrocarbon. Then you also have this unbound hydrocarbon, reacting with oxygen going to CO, which is again incomplete combustion. That means it does not go to CO2. You have nitrogen from the air now which can combine at this high temperature to give rise to oxides of nitrogen represented as NOx and under this NOx umbrella. We have NO, which is nitric oxide and NO2, which is nitrogen dioxide.
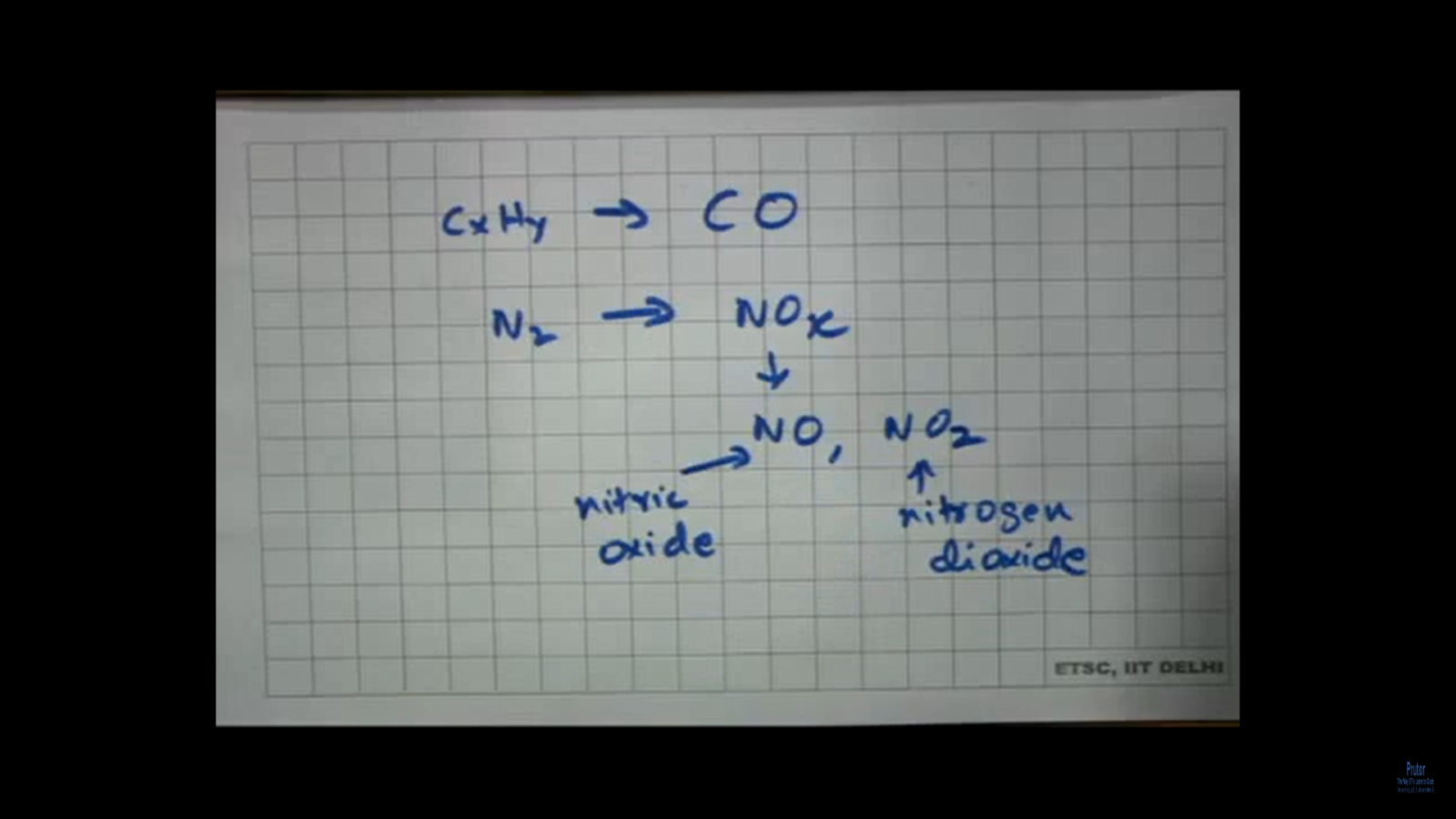 So, then, in one shot, I can write that if I have air +petrol, that is what you are burning, will give rise to CO2 + H2O, you know these are the ideal ones, + CO + NOx. These are the ones which we do not want, + unburnt hydrocarbons. The main problem arises from these three, and that is why these three are often clubbed as pollutants or environment. Sorry, this should read as in environmental pollutants. That means they pollute the environment. So, see you are talking about fuel burning right, you are talking about an ideal combustion where I should be getting ideally: carbon dioxide and water. I do not have much to worry about, but then because combustion is not ideal because of the conditions there will be some hydrocarbons which should not be burnt. There would be carbon which would be incompletely oxidized. That means it would not go to carbon dioxide. It would rather go to carbon monoxide this carbon coming from the hydrocarbon, and then you have so much nitrogen in air. So, this nitrogen can combine at this high temperature with oxygen to give rise to different oxides, you know NOx under which we have NO and NO2.
So, then, in one shot, I can write that if I have air +petrol, that is what you are burning, will give rise to CO2 + H2O, you know these are the ideal ones, + CO + NOx. These are the ones which we do not want, + unburnt hydrocarbons. The main problem arises from these three, and that is why these three are often clubbed as pollutants or environment. Sorry, this should read as in environmental pollutants. That means they pollute the environment. So, see you are talking about fuel burning right, you are talking about an ideal combustion where I should be getting ideally: carbon dioxide and water. I do not have much to worry about, but then because combustion is not ideal because of the conditions there will be some hydrocarbons which should not be burnt. There would be carbon which would be incompletely oxidized. That means it would not go to carbon dioxide. It would rather go to carbon monoxide this carbon coming from the hydrocarbon, and then you have so much nitrogen in air. So, this nitrogen can combine at this high temperature with oxygen to give rise to different oxides, you know NOx under which we have NO and NO2.
 So, why are these referred to as pollutants? Now, before I write something else down, let me show you something as a picture now, if you look at this picture, if you look at this picture and if you look at my or the white pointer, what you see is at the top of this picture, its written photochemical smog. I will come to that word later or those two words later, but remember this smog, which means that you have heavy pollutants in the air. Now look at the picture below and what you see is not only do you see so many cars running, but if you look at the atmosphere, it’s very hazy. No way can you say that it is clean air that you are breathing its very hazy. It is hazy, why one of the main reasons why we have pollutants is the emission coming out from cars. So, then I can write about emission from cars are a huge source of environmental pollution. It is a huge source. Ok.
Now, what do cars do to stop this? So, let us look at a picture of a car. So, if you look at this car again, if you follow my arrow, you can see its written as so. This is the skeleton of a car, and you are seeing some components inside. I will tell you which are the main components right now, we will be discussing about, are relevant for our discussion. If you look at this one, this is called the exhaust manifold exhaust manifold means nothing that when the engine is driving, your hydrocarbon is burning. That means your fuel is burning. Then, whatever gases you produce, they come out through these exhaust pipes. Ok, so this is what is exhaust pipe A all these gases come out to the exhaust pipes. Now, if you would not do anything to these gases, then what would happen is these exhaust gases would go straight out into the air and pollute your environment, but that is a big, no, no right, because pollution is very harmful for us and in big cities. It is directly related to big cities, the more the number of cars you have, the greater number of automobiles you have, the greater the pollution. So, then each and every car has to do something about it, and this is compulsory.
So, what do cards do so each, and every car is equipped with something referred to as a catalytic converter referred to as a catalytic converter. If you see my pointer or arrow, I am moving this pointer over this word catalytic then converter do not worry about the three- way, but what the catalytic converter is supposed to do is it is supposed to take these gases, the harmful ones and convert them to non-harmful ones so that when, finally, the gases come out through this pipe, you can see out here, exhaust pipe tip, then these pollutants, like NOx, CO and the unburned hydrocarbons, are not there. So, this is one of the most important features, one of the most important features environmentally, that a car has to have such that pollution of the environment is kept to a minimum.
So, why are these referred to as pollutants? Now, before I write something else down, let me show you something as a picture now, if you look at this picture, if you look at this picture and if you look at my or the white pointer, what you see is at the top of this picture, its written photochemical smog. I will come to that word later or those two words later, but remember this smog, which means that you have heavy pollutants in the air. Now look at the picture below and what you see is not only do you see so many cars running, but if you look at the atmosphere, it’s very hazy. No way can you say that it is clean air that you are breathing its very hazy. It is hazy, why one of the main reasons why we have pollutants is the emission coming out from cars. So, then I can write about emission from cars are a huge source of environmental pollution. It is a huge source. Ok.
Now, what do cars do to stop this? So, let us look at a picture of a car. So, if you look at this car again, if you follow my arrow, you can see its written as so. This is the skeleton of a car, and you are seeing some components inside. I will tell you which are the main components right now, we will be discussing about, are relevant for our discussion. If you look at this one, this is called the exhaust manifold exhaust manifold means nothing that when the engine is driving, your hydrocarbon is burning. That means your fuel is burning. Then, whatever gases you produce, they come out through these exhaust pipes. Ok, so this is what is exhaust pipe A all these gases come out to the exhaust pipes. Now, if you would not do anything to these gases, then what would happen is these exhaust gases would go straight out into the air and pollute your environment, but that is a big, no, no right, because pollution is very harmful for us and in big cities. It is directly related to big cities, the more the number of cars you have, the greater number of automobiles you have, the greater the pollution. So, then each and every car has to do something about it, and this is compulsory.
So, what do cards do so each, and every car is equipped with something referred to as a catalytic converter referred to as a catalytic converter. If you see my pointer or arrow, I am moving this pointer over this word catalytic then converter do not worry about the three- way, but what the catalytic converter is supposed to do is it is supposed to take these gases, the harmful ones and convert them to non-harmful ones so that when, finally, the gases come out through this pipe, you can see out here, exhaust pipe tip, then these pollutants, like NOx, CO and the unburned hydrocarbons, are not there. So, this is one of the most important features, one of the most important features environmentally, that a car has to have such that pollution of the environment is kept to a minimum.
 Ok, now what you see in this picture it assumed into figure of this catalytic converter. So, typically, if you have access to a car or if you see your neighbors have cars, your friends have cars and if you look at the bottom of the car, you will see an object like this very much so like this. You know not too much difference in design, but the catalytic converters of most of the cars would have this structure. Now, let us decide, or let us look at what the catalytic converter does? See is by the name what does it mean by the name? If I say the name, catalytic converter, then by the name, it suggests that I am converting something. What am I converting here? I am converting the gases under NOx, then I am converting carbon monoxide and I am converting unburnt fuel. How am I doing it? Because it is called a catalytic converter, I say it is doing with the help of catalyst.
Now, if you go back to one of our discussions before you know when we are slowly moving into this concept of chemical kinetics, and we said that as opposed to thermodynamics, chemical kinetics tells you about the rate of the reaction and also about some idea of what goes on during the reaction, then, a question that comes to your mind automatically is: can I control the rate and we discussed that it can be concentration, say one. It can be temperature second, and it can also be a catalyst which ends up changing the rates of reactions. So, that means this catalytic converter would be having some catalysts either one catalyst or a combination of catalysts, which we will just see which will help converting these harmful pollutants to something which would not harm us or pollute the environment. And with the idea, and with the fact that the number of cars on roads, the number of auto mobiles, not only cars, trucks, motorcycles, everything on bikes on road are increasing day by day. It just makes sense that this level of pollution coming out or being contributed to by these automobiles would increase if measures were not taken to control that level of pollutants being emitted through the exhaust pipe of the cars. Ok.
Ok, now what you see in this picture it assumed into figure of this catalytic converter. So, typically, if you have access to a car or if you see your neighbors have cars, your friends have cars and if you look at the bottom of the car, you will see an object like this very much so like this. You know not too much difference in design, but the catalytic converters of most of the cars would have this structure. Now, let us decide, or let us look at what the catalytic converter does? See is by the name what does it mean by the name? If I say the name, catalytic converter, then by the name, it suggests that I am converting something. What am I converting here? I am converting the gases under NOx, then I am converting carbon monoxide and I am converting unburnt fuel. How am I doing it? Because it is called a catalytic converter, I say it is doing with the help of catalyst.
Now, if you go back to one of our discussions before you know when we are slowly moving into this concept of chemical kinetics, and we said that as opposed to thermodynamics, chemical kinetics tells you about the rate of the reaction and also about some idea of what goes on during the reaction, then, a question that comes to your mind automatically is: can I control the rate and we discussed that it can be concentration, say one. It can be temperature second, and it can also be a catalyst which ends up changing the rates of reactions. So, that means this catalytic converter would be having some catalysts either one catalyst or a combination of catalysts, which we will just see which will help converting these harmful pollutants to something which would not harm us or pollute the environment. And with the idea, and with the fact that the number of cars on roads, the number of auto mobiles, not only cars, trucks, motorcycles, everything on bikes on road are increasing day by day. It just makes sense that this level of pollution coming out or being contributed to by these automobiles would increase if measures were not taken to control that level of pollutants being emitted through the exhaust pipe of the cars. Ok.
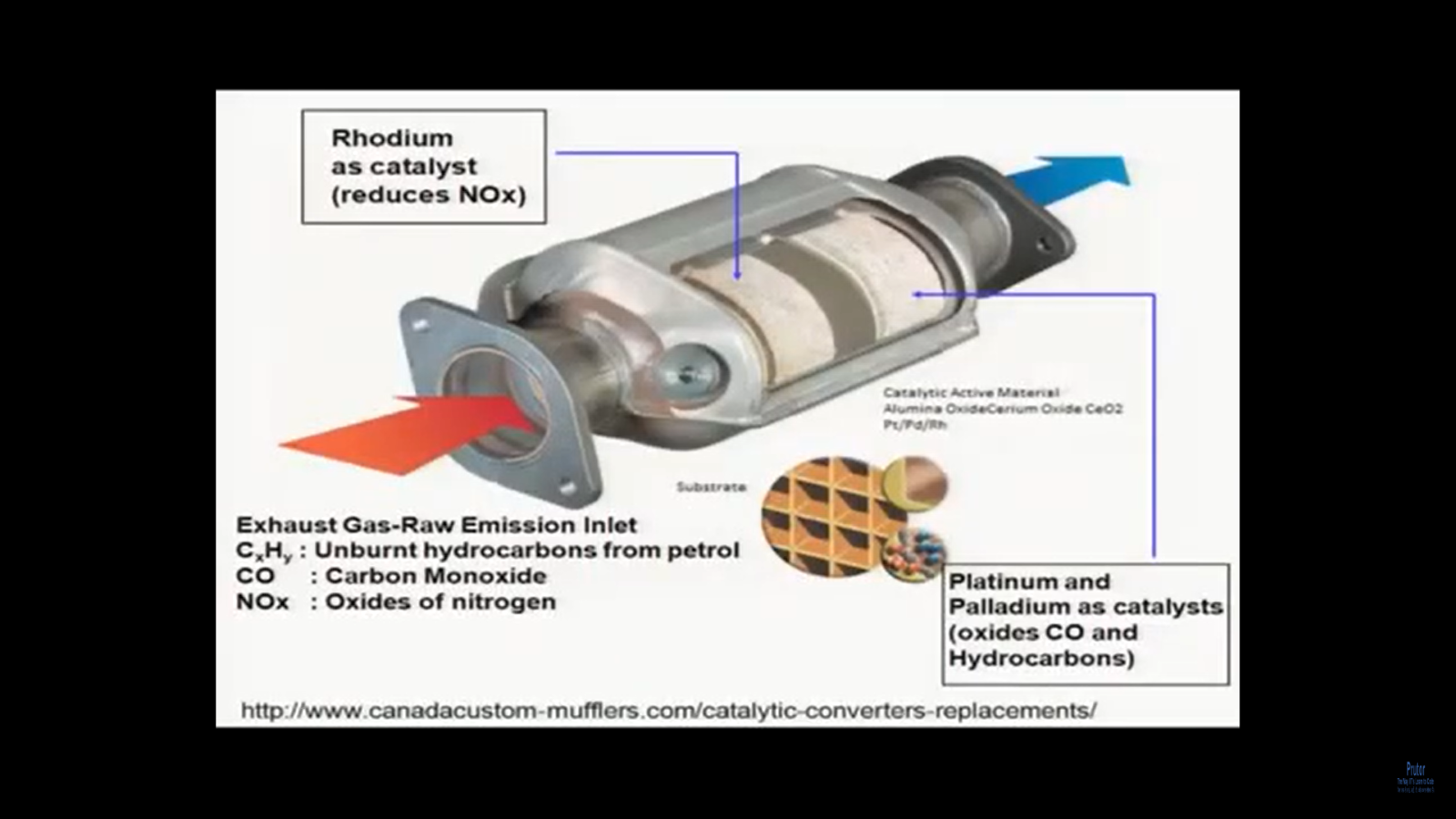
 So, here, as we were talking, remember we looked at this catalytic converter right now. What I am going to show you is the inside of a catalytic converter. Now there is a reason we are going to this because we do understand that this is we are talking about chemistry in something as modern technology as cars, with the technology improving day by day. So, now, if you look at the inside of this catalytic converter, what do we see? So, the construction is very simple. On the two sides, you have two ports: what are these ports? If you see this red arrow, you follow. You follow my white arrow. If you see this big red arrow, then this is the inlet port, so the inlet pipe. What does it do? It comes from the exhaust manifold where the gases are produced after burning on fuel. So, then you can see you have those unburned CxHy, then carbon monoxide then oxides of nitrogen, which would enter the catalytic converter to this port.
Ok now the inside of the catalytic converter, you can see there are two slabs now without going into the very details, also realize one thing that these slabs are built with certain high temperature materials which can resist the temperature at which this fuel is being burnt, so that those do not get bad or not affected, but not only that in these slabs you have catalysts embedded so, for example, the first slab you can see out here. This slab has rhodium as a catalyst. What does rhodium do? As it is said out here, rhodium as a catalyst, reduces the oxides of nitrogen. What is it reduced to, so NOx gets transformed to nitrogen and oxygen. So, what does rhodium do? That means rhodium is reducing NOx oxide of nitrogen to nitrogen and oxygen gases. So, that’s why rhodium is the catalyst now also, if you look at this small circle, which is a part of this catalyst, so what happens is the way this catalyst is made or the way this you know this. This structure is made where the rhodium catalyst is there. It is full of, it is porous. That means it is full of pores.
Why do you need pores? You need pores so that the gas which is coming out of the gases which coming out from the exhaust pipe or through the exhaust pipe from the exhaust manifold, can pass through this one during passing. What is happening is these are getting reduced at least NOx. In this case the oxidizer is normally reduced to nitrogen and oxygen now comes the next one. Remember you have been able to take care of the oxides of nitrogen, but what are you left with? You are still left with, remember, still left with carbon monoxide gas and then the incompletely burnt hydrocarbons. What do you do here? So, in the second slab or structure what you have is you have two catalysts: what are these catalysts? The second one, the two catalysts are as shown so platinum and palladium. What do they do? They should be oxidizing, so they oxidize carbon monoxide and hydrocarbons.
So, here, as we were talking, remember we looked at this catalytic converter right now. What I am going to show you is the inside of a catalytic converter. Now there is a reason we are going to this because we do understand that this is we are talking about chemistry in something as modern technology as cars, with the technology improving day by day. So, now, if you look at the inside of this catalytic converter, what do we see? So, the construction is very simple. On the two sides, you have two ports: what are these ports? If you see this red arrow, you follow. You follow my white arrow. If you see this big red arrow, then this is the inlet port, so the inlet pipe. What does it do? It comes from the exhaust manifold where the gases are produced after burning on fuel. So, then you can see you have those unburned CxHy, then carbon monoxide then oxides of nitrogen, which would enter the catalytic converter to this port.
Ok now the inside of the catalytic converter, you can see there are two slabs now without going into the very details, also realize one thing that these slabs are built with certain high temperature materials which can resist the temperature at which this fuel is being burnt, so that those do not get bad or not affected, but not only that in these slabs you have catalysts embedded so, for example, the first slab you can see out here. This slab has rhodium as a catalyst. What does rhodium do? As it is said out here, rhodium as a catalyst, reduces the oxides of nitrogen. What is it reduced to, so NOx gets transformed to nitrogen and oxygen. So, what does rhodium do? That means rhodium is reducing NOx oxide of nitrogen to nitrogen and oxygen gases. So, that’s why rhodium is the catalyst now also, if you look at this small circle, which is a part of this catalyst, so what happens is the way this catalyst is made or the way this you know this. This structure is made where the rhodium catalyst is there. It is full of, it is porous. That means it is full of pores.
Why do you need pores? You need pores so that the gas which is coming out of the gases which coming out from the exhaust pipe or through the exhaust pipe from the exhaust manifold, can pass through this one during passing. What is happening is these are getting reduced at least NOx. In this case the oxidizer is normally reduced to nitrogen and oxygen now comes the next one. Remember you have been able to take care of the oxides of nitrogen, but what are you left with? You are still left with, remember, still left with carbon monoxide gas and then the incompletely burnt hydrocarbons. What do you do here? So, in the second slab or structure what you have is you have two catalysts: what are these catalysts? The second one, the two catalysts are as shown so platinum and palladium. What do they do? They should be oxidizing, so they oxidize carbon monoxide and hydrocarbons.
 So, the next slab, which is the second one out here, has as catalysts platinum and palladium right. They oxidize CO and CxHy, ok, so that means CO + O2 gas gives me CO2 gas right and also remember from before CxHy + O2 gas. So, this is also gas would be giving me CO2 + H2O gas. Ok, so if I balance this, this is how it would come. So, what has this catalytic converter done for you? What this catalytic converter has done is it has taken these harmful gases, the first being oxidized of nitrogen, which were reduced to nitrogen and oxygen no pollutants, then the ones which are coming out - carbon monoxide and the hydrocarbons they are now being oxidized using platinum and Palladium to carbon dioxide and water. By this way, and by efficient design of the catalytic converter, you can try to minimize you can try to minimize the extent of harmful pollutants that are being given out by the car or are coming out from you can see this blue, solid arrow: this is the other side of your catalytic converter, to which the gases which just got converted or some percentage we did not get converted go through.
So, this is really fascinating right. So, within that short time - and I will tell you what typically the time is within the short time - the car engine is running, the fuels are being burnt. You know these pollutants are being produced. These pollutants are sent to the exhaust pipe into the catalytic converter. They are passing through the catalytic converter during that time. Simultaneously what is happening is the nitric oxides, oxygen nitrogen is getting reduced and CxHy and carbon monoxide these are getting oxidized to less harmful or non-polluting species.
So, the next slab, which is the second one out here, has as catalysts platinum and palladium right. They oxidize CO and CxHy, ok, so that means CO + O2 gas gives me CO2 gas right and also remember from before CxHy + O2 gas. So, this is also gas would be giving me CO2 + H2O gas. Ok, so if I balance this, this is how it would come. So, what has this catalytic converter done for you? What this catalytic converter has done is it has taken these harmful gases, the first being oxidized of nitrogen, which were reduced to nitrogen and oxygen no pollutants, then the ones which are coming out - carbon monoxide and the hydrocarbons they are now being oxidized using platinum and Palladium to carbon dioxide and water. By this way, and by efficient design of the catalytic converter, you can try to minimize you can try to minimize the extent of harmful pollutants that are being given out by the car or are coming out from you can see this blue, solid arrow: this is the other side of your catalytic converter, to which the gases which just got converted or some percentage we did not get converted go through.
So, this is really fascinating right. So, within that short time - and I will tell you what typically the time is within the short time - the car engine is running, the fuels are being burnt. You know these pollutants are being produced. These pollutants are sent to the exhaust pipe into the catalytic converter. They are passing through the catalytic converter during that time. Simultaneously what is happening is the nitric oxides, oxygen nitrogen is getting reduced and CxHy and carbon monoxide these are getting oxidized to less harmful or non-polluting species.
 Ok, now, if you go by the time, ah that it takes so because we are talking about kinetics, it is always you know well to give you some sense of time, so the time for which you know it kind of stays in contact. You know, if you think about how fast this whole process happens or how fast or how long does these remain, you know these gases remain in contact with the catalyst? Then it takes about fifty to seventy milliseconds. So, if I can write out here, see takes about fifty to seventy milliseconds for the gas to go through the converter right. Remember, the car is running so ‘ms’ stands for milliseconds and during this time this whole conversion has to take place. So, you realize that it is not only about the reaction that is happening, understand, conditions, reaction or reactions when the temperature is raised because your burning fuel and so on. But it is also that in the catalytic converter, when the gases are passing through the two slabs, where you had these catalysts for a very short time, the gases get the opportunity to pass over the catalysts or in other words the catalysts, have only that much amount of time to make sure that the conversion can happen as efficiently as possible.
Ok, now, based on this, if you read newspapers, then you would come across some guidelines. What are those guidelines in terms of environmental pollution? One very common guideline for automobiles is going by that Bharath stage 4. What does this mean? What it means is that, under this, every car say has to comply by the restrictions imposed under this concept, or this heading Bharat stage 4. What is it related to? It is directly related to the pollutants or the amount of these pollutants which are coming out through your exhaust. So, in the coming days you would see that cars would have to comply by say Bharath stage 6 that means the amount of carbon monoxide that can come out through the exhaust, which was not oxidized to carbon dioxide, has to be even lower than what is permissible now or the amount of oxides of nitrogen that can come out permissible amount would be much lower than what is being used now, which is part stage four right. So, this was one example were chemistry in cars, along with the rates of the reaction, high temperature high rates right, then, because of burning of fuel, then also the application of catalyst. Everything is happening together right. That is why chemical kinetics is such an important concept.
So, what we will do in the next lecture is again before you know we delve into the real equations about the rate of chemical reactions and so on. We will look at another example and if you can work on that yourself or think about that yourself, I will tell you what the example is. That example is about airbags, a safety feature in cars, and I will tell you how or what fascinating chemistry goes on in there as a direct relevance to our discussion on chemical kinetics. Thank you.
Ok, now, if you go by the time, ah that it takes so because we are talking about kinetics, it is always you know well to give you some sense of time, so the time for which you know it kind of stays in contact. You know, if you think about how fast this whole process happens or how fast or how long does these remain, you know these gases remain in contact with the catalyst? Then it takes about fifty to seventy milliseconds. So, if I can write out here, see takes about fifty to seventy milliseconds for the gas to go through the converter right. Remember, the car is running so ‘ms’ stands for milliseconds and during this time this whole conversion has to take place. So, you realize that it is not only about the reaction that is happening, understand, conditions, reaction or reactions when the temperature is raised because your burning fuel and so on. But it is also that in the catalytic converter, when the gases are passing through the two slabs, where you had these catalysts for a very short time, the gases get the opportunity to pass over the catalysts or in other words the catalysts, have only that much amount of time to make sure that the conversion can happen as efficiently as possible.
Ok, now, based on this, if you read newspapers, then you would come across some guidelines. What are those guidelines in terms of environmental pollution? One very common guideline for automobiles is going by that Bharath stage 4. What does this mean? What it means is that, under this, every car say has to comply by the restrictions imposed under this concept, or this heading Bharat stage 4. What is it related to? It is directly related to the pollutants or the amount of these pollutants which are coming out through your exhaust. So, in the coming days you would see that cars would have to comply by say Bharath stage 6 that means the amount of carbon monoxide that can come out through the exhaust, which was not oxidized to carbon dioxide, has to be even lower than what is permissible now or the amount of oxides of nitrogen that can come out permissible amount would be much lower than what is being used now, which is part stage four right. So, this was one example were chemistry in cars, along with the rates of the reaction, high temperature high rates right, then, because of burning of fuel, then also the application of catalyst. Everything is happening together right. That is why chemical kinetics is such an important concept.
So, what we will do in the next lecture is again before you know we delve into the real equations about the rate of chemical reactions and so on. We will look at another example and if you can work on that yourself or think about that yourself, I will tell you what the example is. That example is about airbags, a safety feature in cars, and I will tell you how or what fascinating chemistry goes on in there as a direct relevance to our discussion on chemical kinetics. Thank you.










