Unit 17 Dual Nature Of Matter And Radiations
Learning Objectives
After going through this unit, you will be able to understand, appreciate and apply the following concepts:
-
Discovery of the phenomenon of photoelectricity.
-
The importance of the experiments of Hallwache, Elster, Gaitel and Leonard.
-
The failure of the classical e.m. theory in explaining the experimental results of photoelectricity.
-
The introduction of the ‘photon theory’ of light, by Einstein.
-
The mechanism of interaction of light, with the free electrons of the metal, in terms of the photon theory of light.
-
Einstein’s photoelectric equation and its success in explaining the experimental results on photoelectricity.
-
The concept of the threshold frequency / wavelength, for a given photosensitive surface.
-
The “dual nature of light”.
-
The extension of the concept of “dual nature” to matter.
-
The concept of “matter waves”, or “deBroglie waves”.
-
The basic expression for the wavelength (the deBroglie wavelength) associated with a particle.
-
The dependence, of the deBroglie wavelength, for a charged particle (like the electron), on the ‘potential’ used to accelerate it.
-
The Davisson and Germer experiment and its role in providing the required (experimental) support to the concept of matter waves.
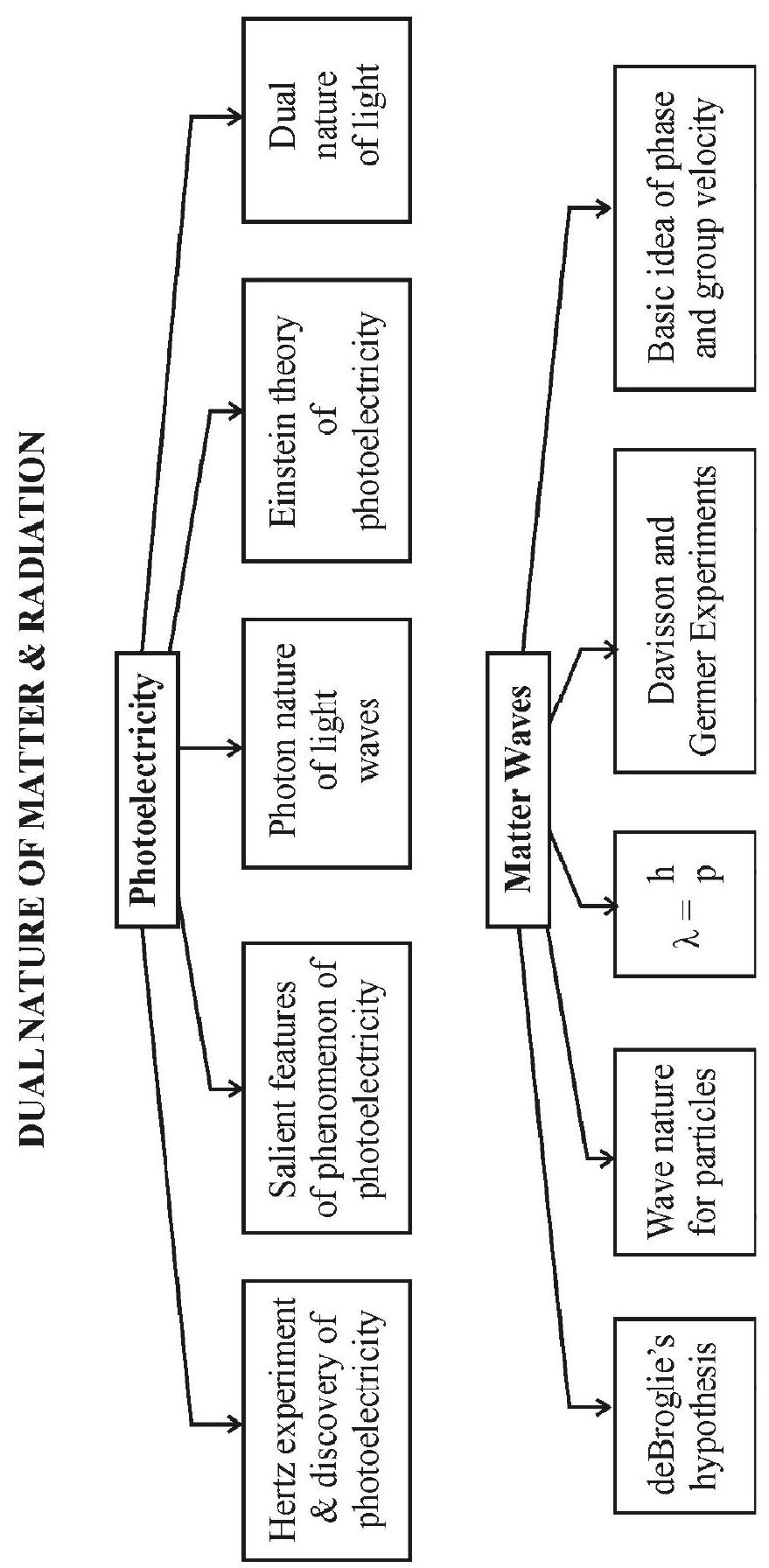
The Photoelectric Effect
The phenomenon of photoelectricity was accidently discovered by Hertz. He was performing an experiment to test Maxwell’s electromagnetic theory. He by means of a spark dischange, was investigating production of electromagnetic waves. Hertz observed that high voltage spark across the detector loop were enhanced when the emitter plate was illuminated by ultraviolet light. It was prabably due to emission of charged particles from the plate due to incidence of ultraviolet radiction. Leonard then determined the e/m value of these particles and showed that they were electrons. Hallwachs, Elster and Giatel then experimentally established that photoemission depend upon.
(i) the nature of the emitter
(ii) the quality of the light used.
By a simple experimental arrangement shown in the figure the following sailent features of the phenomenon of photoelectric emission was established.
Sailent Features of Phenomenon of Photoelectricity
1. Photoelectric emission is an instanteous phenomenon.
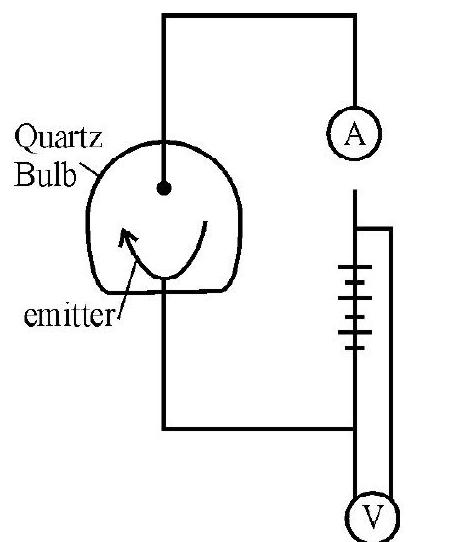
2. A photoemitter shows the photoelectric effect only if the incident radiation has a frequency above a certain value called the threshold frequency. This frequency is a characterstics of the photoemitter. Below threshold frequency no emission is possible whatever may be the intensity of light or whatever be the time span for which the light is incident on the photoemitter.
3. For a given frequency (above threshold) the number of photo electrons emitted is directly proportional to the intensity of light.
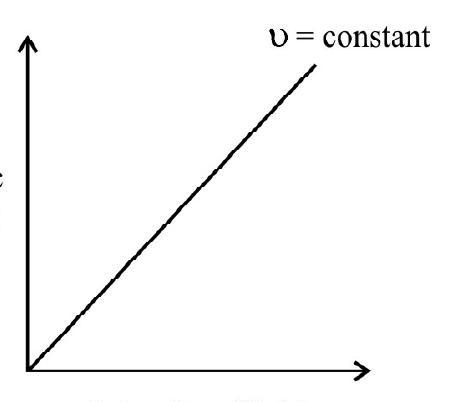
4. The maximum energy of the emitted photoelectrons is directly proportional to the frequency of incident light and has nothing to do with the intensity of light.
The maximum kinetic energy of the emitted photoelectrons from a photoemitter is measured by determining the stopping or retarding potential.
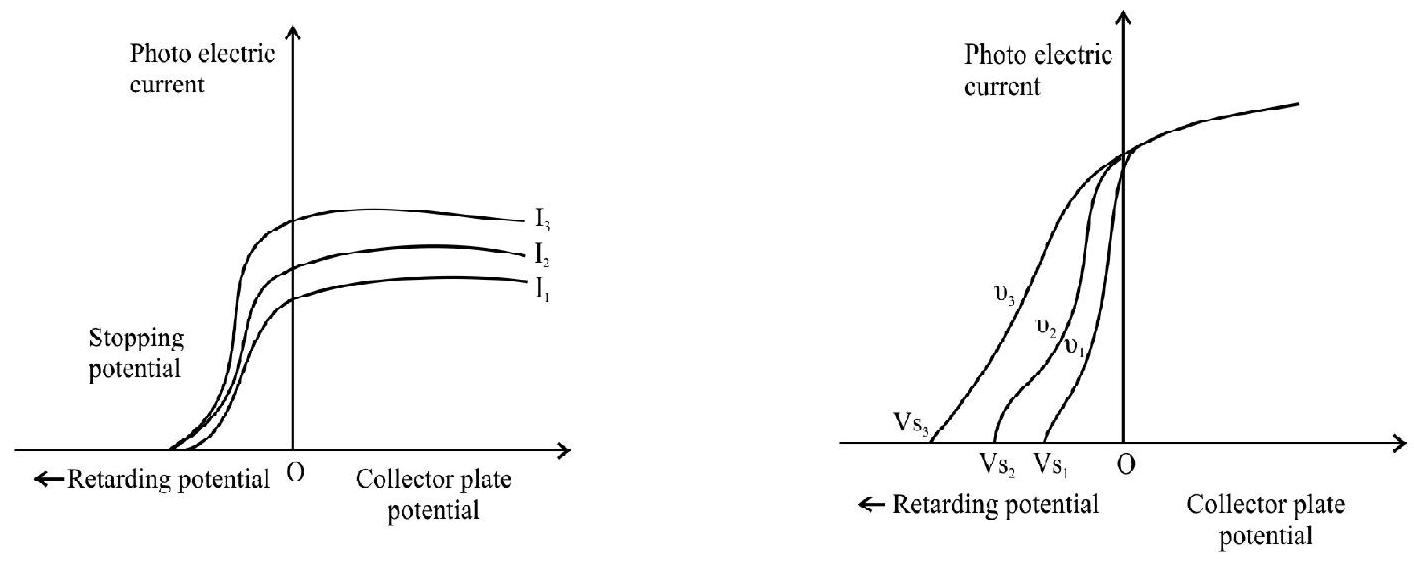
Variation of photoelectric current with collector plate potential for different frequencies of incident radictions (for same intensity)
5. There exists a energy distribution among the emitted photoelectrons from a photoemitter.
Failure of Electromagnetic Theory of Light
The above mentioned features of the phenonmenon of photoelectriciy were attempted to be explained by electromagnetic theory. However except fot the fact that electric and magnetic field associated with an electromagnetic wave can cause the emission of photoelectrons, the electromagnetic theory could not explain even a single fact concerning the observed features of the phenomenon.
It is natural to expect that the energy transmitted by light should smoothly and evenly distritube itself in the space. If it is so than each electron would intercept only an significant small amount of energy and would require a long time (may be days or years) to accumulate enough energy to escaope from the metal. Experimentally however it is observed that the photoemission is instantaneous.
Again on the basis of electromagnetic theory there should not be any thing like threshold frequency existing. There seemed to be no reason why sufficiently intense beam of low frequency liget would not be able to produce photoelectricity if low intensity radiation of higher frequency can produce it.
On classical theory we expect that light of high intensity, consisting of strong electric and magnetic fields, would give higher kinetic energy to the liberated electrons. This however does not happen. Also the electron’s energy should not depend particularly on the light frequency but it does.
Thus classical electromagnetic theory is not able to explain all the sailent features of the phenonmenon of photoelectricity.
Einstein’s Theory
The challange passed by photoelectricity was met by Einstein. Einstein’s took his clue from Planck’s explanation of black body spectrum. Einstein’s assumed that light consists of quantum packets of energy,
the energy of a packet is
The above equation is known as Einstein’s equation and is fundamental in explaining the facts of the phenomenon of photoelectricity.
1. According to Einstein’s theory the emission of electrons from a photoemitter is concieved as a collision process between a photon and electron and hence is a instantaneous phenomenon.
2. According to Einstein’s theory a more intense beam incident on a metal surface implies that more photons are being incident on the metal surface per unit area per unit time. Each photon involves itself a collision with an electron and consequently is emitted. The more is the number of incident photons the more will the number of collisions occuring and more is the number of photoelectrons emitted. Hence at a given appropriate frequency the photoelectric current is directly proportional to the intensity of light.
3. There exists a threshold frequency. We know that the quantum of energy of a photon is equal to
4. According to Einstein’s equation
it is obvious that the maximum kinetic energy of the emitted photoelectron with depend linealy on the frequency of incidence light and has nothing to do with the intensity of light.
In terms of concept of stopping potential. we can also write
5. The existance of energy distribution among the emitted electrons is explained as follows. In writing the relation
we assume that the emission take place at the surface of the emitter. However if a photon electron collision take place inside a emitter, the emitted electron will lose some energy due to collisions with the atoms in the lattice of the emitter and hence in this case Einstein’s equation will become
where
Dual Nature of Light
The classical electromagnetic theory of light is very successful in explaining the phenomenon of interference. diffraction, polarisation etc. In fact these phenomenon can only be explained by this theory. However the electromagnetic theory was a complete failure in explaining the experimental facts of photoelectricity. Later on it was found that electromagnetic theory cannot explain the phenomenon of “Compton scattering” and “specific heat of solids”. One has to introduce the concept of photons (particles) to explain the above mentioned phenomenon. Naturally the questions arieses What is Light? Is it a wave entity or Is it a particle. The answer to this question is either or neither. We now regard light to have a dual nature. It mainfest itself as a wave in phenomenon like interference, diffraction, polarisation etc. while it shows photon (particle) behaviour in the phenomenon of photoelectricity and Compton scattering. However light can never exhibit both wave and particle behaviour simulataneously. These facts are further developed with astonishing results in the studies of Quantum Mechanics.
Example-1
Ultraviolet light of wavelength
(i) the maximum kinetic energy of the photoelectrons
(ii) the stopping potential
Show Answer
Solution:
(i) Maximum kinetic energy is given by
(ii) Stopping potential
Example-2
Monochromatic radiation of wave length
Show Answer
Solution:
We have
For neon;
Again using the relation
Example-3
The work function for different metals given, below is
Which of these metals will not give photoelectric emission for a radiation of
Show Answer
Solution:
Energy of incident radiation
From the values given in question it is obvious that photoemission will take place from
Bringing laser beam nearer and nearer will only increases the intensity of incident radiation. So the photoelectric current from
Example-4
The work function of caesium is
Show Answer
Solution:
(a) Let
(b)
Example-5
The above table gives the values of stopping potential which were obtained in a photoelectric experiment which was performed using calcium as emitter.
From this data, obtain the value of the Plank’s constant.
Show Answer
Solution:
The plot of
Slope of the graph is
Matter Waves
The phenomenon of black body radiations could not be explained satisfactarly on the basis of classical theories of radiations till Max Planck’s introduced the radically, new concept of Quantisation. Later on the study of the phenomenon of photoelectricity and the failure of the classical electromagnetic theory in explanning the various features once again forced the scientists to take resort to the newly introduced Photon theory of light. Close to the heels of these phenomeon came a number of other discoveries which also required one to adopt the photon theory of radiations (X-ray scottering is a prominentexample). However the well established phenomeon of interference diffraction, polarisation etc. required one to adopt a wave picture of light. Ultimately light was given a sort of Dual Nature which sometimes manifest itself as waves and sometimes as particles i.e. photons.
Following the dual nature of light deBroglie suggested to think in terms of the same dual nature for Matter or particles. Arguing that nature would prefer symmatery, he made the bold assertion that just as radiation can sometime behave like particle, the matter should also sometimes behave as waves. Thus the idea of matter waves was born.
The deBroglie Formula
The deBroglie formula for calculating the wavelength to be associated with any material particle is analogous to the one for radiations. A photon of light frequency
deBroglie asserted that the same formula holds for matter also. Thus the wave length that must be associated with a material particle of momentum
It is obvions that since the value of
Some important considerations regarding beBrogeli waves:
1. If we take into consideration relevestic variation of mass with velocity
The modified deBroglie formula for
2. Concept of Group Velocity
In our discussion so far we associated a single well defined wavelength (monochromatic) with a given particle. However we know that a monochromatic wave is infinite in extent and hence cannot be localized in a well defined region of space. This is contradictory to the observed behaviour of particles which are always well localized. To overcome this diffculty we introduce the idea of a wave packet. This wave packed is formed by superposition of a number of waves situated on and around the central wavelength given by the deBroglie relation. We assume that such a wave packet should have a resultant amplitude which is appreciable different from zero only in a small region of space having the dimensions of particle. The velocity of the component monochromatic waves making up the wave packet is known as Phase velocity (can be
3. Probability Interpretation to Matter Waves
Max Born suggested a probability interpretatation of the matter waves amplitudes. According to this interpretation the intensity (square of amplitude) of the matter wave at a point is determined by the probability density of the particle at that point (probability per unit volume).
4. Davission and Germer Experiment
Davission and Germer experiment was originally desinged to be a study of the scattering of electrons by a nickel crystal.Bascially the experiment consisted in directing a well defined beam of electrons on a nickel crystal and obeserving the scattered electron intensity as a function of the angle with the direction of incidence. As was to be expected they found the intensity to decrease continuously as the angle of scattering increased. However in the course of their experiment, the vaccum system broke drown and the nickel crystal acquired a coat of the oxide. To remove the oxide, the nickel crystal was reduced slowly in a hot oven. The whole system was reset and the experiment repeated. To the utter surprise of everyone, it was found that the scattered electron intensity now went through Maxima and Minima i.e. the electron were being diffracted.
The experimental arrangment used by Davission and Germer is shown in the figure. It consist of an electron gun. The electron gun is a tungsten filament coated with barium oxide. This is heated by a low voltage power supply. The accelerated emitted electrons fall upon a nickel target which is capable of rotation about an axis perpendicular to the plane of page. The scattered electrons are recieved by a moveable Faraday cylinder which had a sat in front of it. One could therefore measure the scattered electron intensity as a function of angle between the incident and scattered beam. From the developments in the field of X-ray diffraction one would expect that corresponding to a set of crystal plane the scattered electron intensity reach a maximum at a particular accelerating potential and at a particular angle of scattering. The polar graphs shown below, confirmed this observation.

The electron diffractions by (111) face of the nickel crystal. These graph show a clear and sharpen defined ‘HUMP’ at an accelerations potential of 54 volt at an angle of
The deBroglie wavelength
We can also calculate
Example-6
X-rays of wavelength
Show Answer
Solution:
Wave length of X-ray used to eject photoelectrons
Frequency
Energy of emitted photoelectron
Example-7
The first order Bragg maximum of electron diffraction in a nickel crystal
Show Answer
Solution:
From Bragg’s law we have
Now,
PROBLEMS FOR PRACTICE
1. A photo sensitive material emits electrons whose maximum velocity is
Show Answer
Answer: 2.559 volt2. The work function for an emitter is
Show Answer
Answer: 2 volt;3. If the photocurrent of a photocell is cut off by a retarding potential of 0.92 volt for monochromatic radiations of
Show Answer
Answer:4. The stopping potential for the fastest electrons from a tantalum surface is
Show Answer
Answer:5. When potasium is radiated with light of wavelength
Show Answer
Answer:6. The ionisation energy of hydrogen atom is
Show Answer
Answer:7. The most energetic electrons emitted from a surface by
Show Answer
Answer:8. Photoelectrons of charge e and constant mass
when applied across the capacitor will enable the electrons to just reach the positive plate.
9. Find the maximum magnitude of the linear momentum of a photoelectron emitted when a light of wavelength
Show Answer
Answer:10. Figure show the plot of the stopping potential versus the frequency of light used in an experiment on photo electric effect. Find (i) the ratio
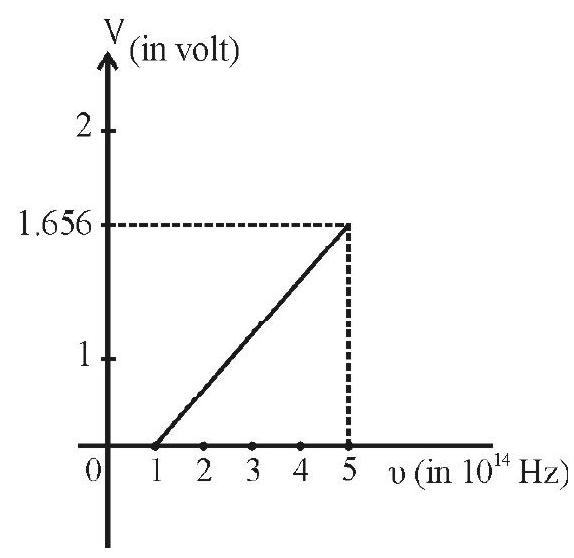
Show Answer
Answer: (i)11. Calculate the deBroglie wavelength associated with an
Show Answer
Answer:12. Calcualte the deBroglie wavelength associated with an electron moving with
Show Answer
Answer:13. The equivalent wavelength of a moving electron is
Show Answer
Answer: 2597 volt14. When 400 volt electrons are diffracted by a crystal the angular diffraction pattern is identical with that produced by X-rays of wavelength
Show Answer
Answer:15. The wavelength of a
Show Answer
Answer:16. Calculate the deBroglie wavelength associated with a sphere of mass
Show Answer
Answer:17. Show that the deBroglie wavlength of an electron.
(i) of energy
and (ii) accelerated through a potential of
Question Bank
Key Learning Points
1. The phenomenon of photoelectricity was discovered during Hertz’s experiment designed to demonstrate the existance electromagnetic waves.
2. On the basis of experimental studies photoelectricity has the following sailent features.
-
Photoelectric emission is an instantneous phenomenon.
-
There exists a threshold frequency below which no emission is possible no matter what may be intensity of illumination.
-
The number of photoelectrons emitted per unit area, per unit time from a emitter is directly proportional to intencity of light.
-
The maximum energy with which electrons are emitted from a emitter is directly proportional to the frequency of incident light but is independent of the intencity of light used.
-
There exists a energy distribution among emitted electrons.
3. The classical electromagnetic theory failed to explain all the above mentioned features of photoelectricity.
4. However one cannot discard electromagnetic theory because then there is noway by which one could explain the phenomenon of interference, diffraction, polarisation dispersion etc.
5. Einstein’s introduced the idea of photons according to which light consists of photons of energy hv. Photons are endavoured as particles moving with velocity of light and having a rest mass of zero.
6. According to Einstein when a photon of energy
This equation is known as Einstein’s photoelectric equation and this successfully explain the sailent features of photoelectricity.
7. In terms of the concept of stopping potential Einstein’s photoequation can be written as
8. Light is assigned a dual character.
9. Since it is a well known fact that nature love symmetery deBroglie argued that if light has to has a duel character, matter particles should also exhibit wave characterstics. He also assigned a wavelength
and predicted that wave characterstics should be found with elementary particles.
10. Davision and Germer accidently obtained a diffraction pattern for accelerated electrons in an experiment designed to study the scattering of electrons by a Nickel crystal in vacuum.
Average
Photons
1. A
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (4)
Solution:
The number of photons emitted by per second by the source is given by
or
Hence option (4) is correct.
Difficult
Photons
2. A photon of
(1) have a energy
(2) have a energy of
(3) have a energy of
(4) will have a energy of
Show Answer
Correct answer: (3)
Solution:
Wave length of the photon is
The electromagnetic wave is near the ultra violet region
Energy of the photon
Hence option (1) is correct.
Average
Photoelectricity
3. Which one of the follownig graphs represent the variation of maximum kinetic energy of photo electrons emitted from a photoemitter with frequency?
(1)
(2)
(3)
(4) D
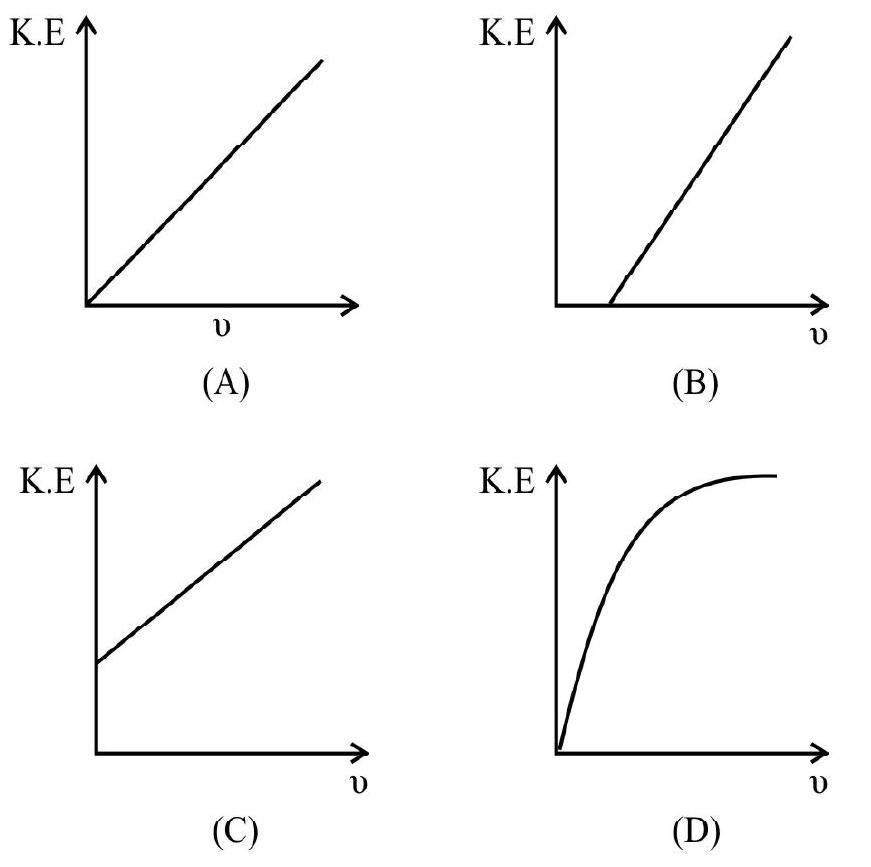
Show Answer
Correct answer: (2)
Solution:
Einstein’s photoelectric equation is
This is of the form
Hence option (4) is correct.
Average
Photoelectricity
4. Which one of the following graphs represent correctly variation of photoelectric current
with the applied voltage?
(1)
(2)
(3)
(4)
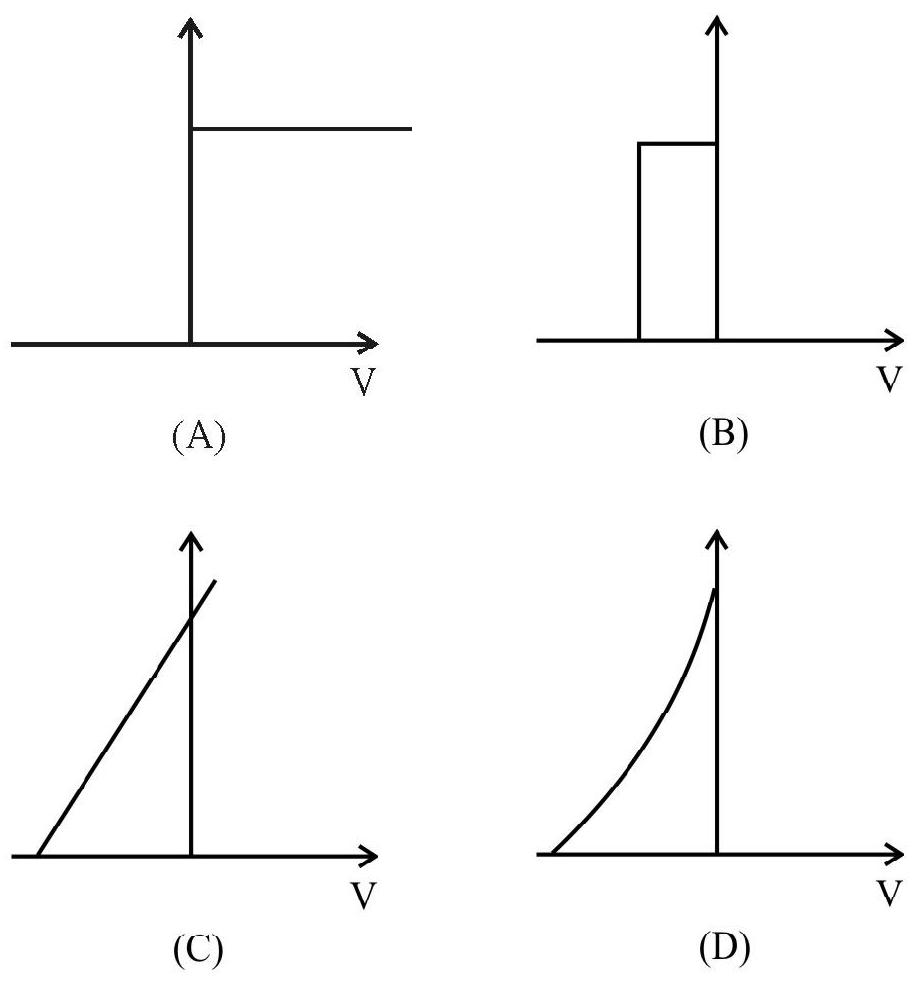
Show Answer
Correct answer: (4)
Solution:
The circuit diagram to study the variation of the photoelectric current with voltage is shown. When the applied potential difference is large all the photo electron emitted by the emitter are collected on the collector. As we decrease the potential difference to zero and then negative values the number of emitted electrons collected by the collector starts decreasing and corresponding to limiting value, called stopping potential the collector is not able to collect anyone of the emitted electrons and photoelectric current tends to become zero. Graph (4) represents this variation.
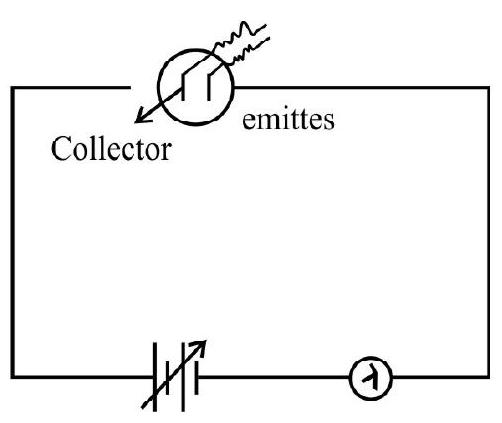
Hence option (4) is correct.
Average
Photoelectricity
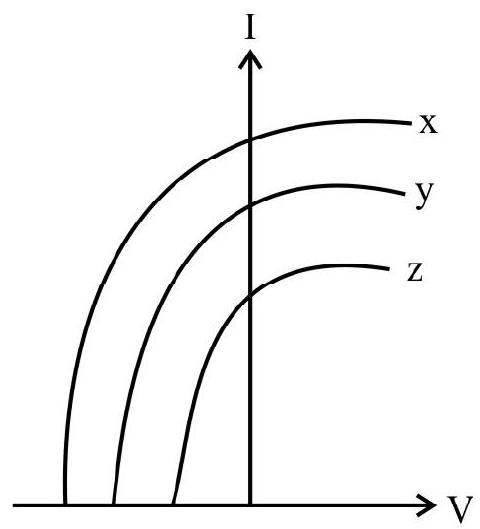
5. For three differenct metal
(1)
(2)
(3)
(4) D
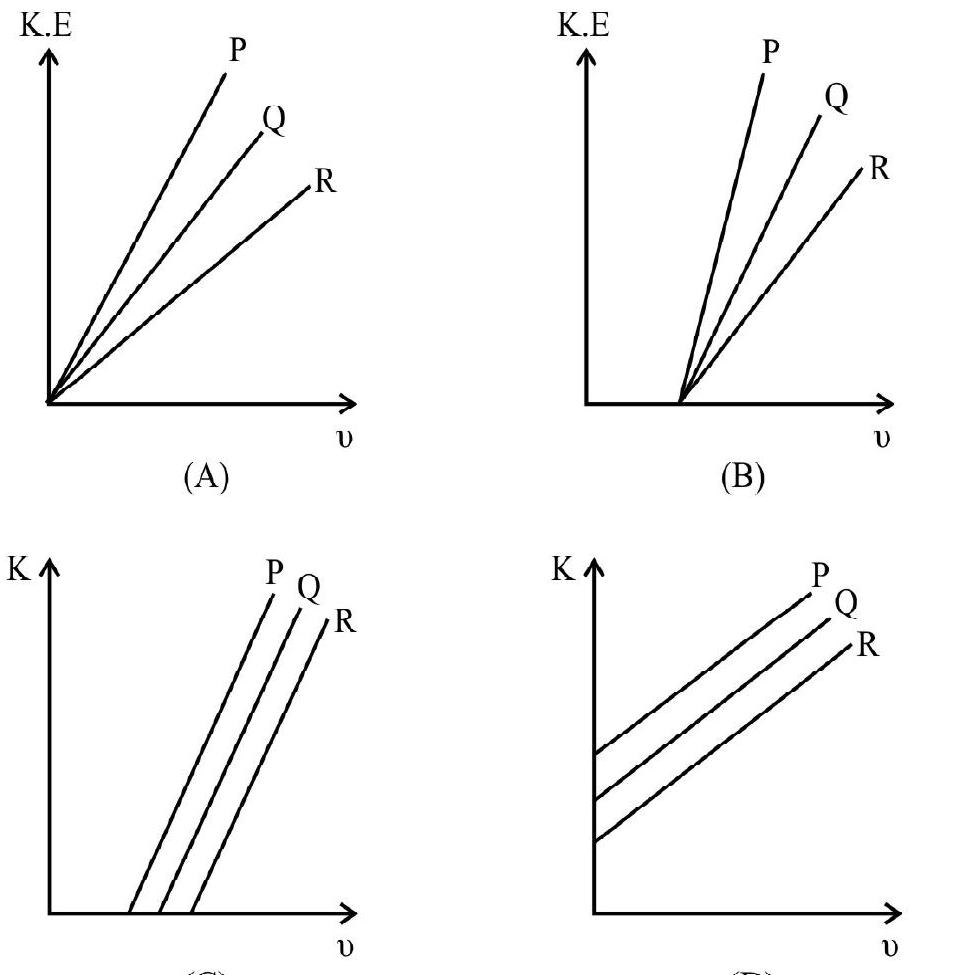
Show Answer
Correct answer: (3)
Solution:
As explained in previous question the metal
Average
Photoelectricity
6. Which one of the following graphs represent correctly the variation of
(1)
(2)
(3)
(4)
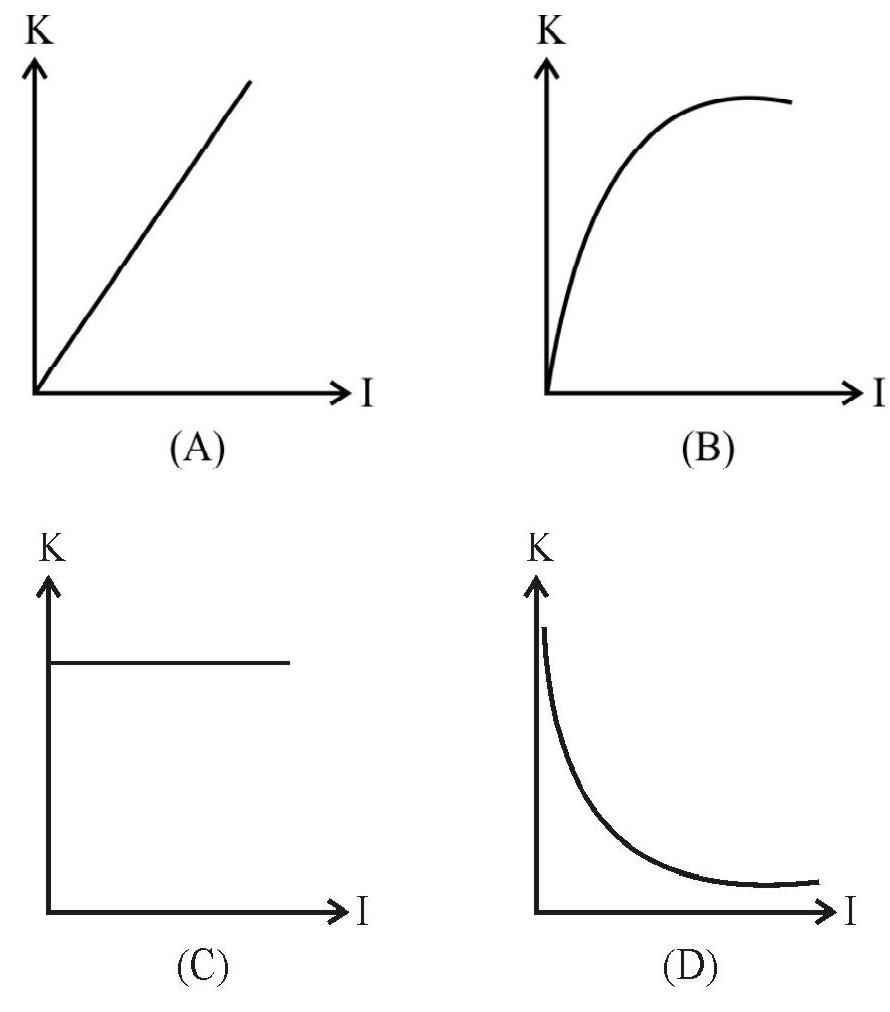
Show Answer
Correct answer: (3)
Solution:
It is an experimental observed fact that the maximum energy with which photoelectrons are emitted does not depend on the intensity of light but depends only on the frequency of the incident light.
Hence option (3) is correct.
Average
Photoelectricity
7. Which one of the following graphs represent correctly the variation of photoelectric current (i) with the intensity (I) of incident radiations?
(1)
(2)
(3)
(4)
(A)
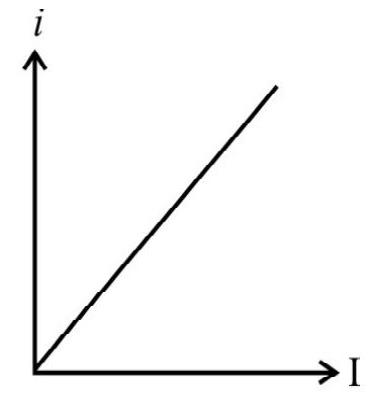
(B)
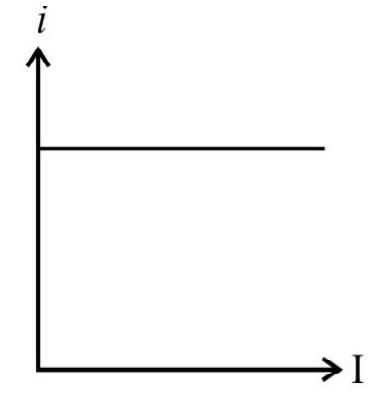
(C)
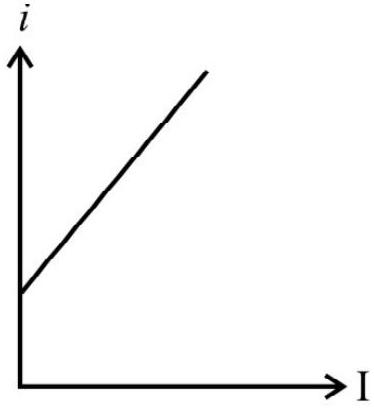
(D)
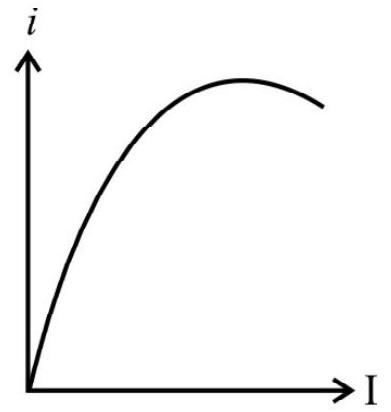
Show Answer
Correct answer: (2)
Solution:
With the increase of intensity of incident light, the number of photons striking the emitter surface per unit area per unit time increases. As each photon colliding and emitting one electron, the number of emitted electrons increases with increase in the increase of intensity of light. The graph in option (2) is representing this fact and therefore is the correct answer.
Hence option (2) is correct.
Average
Photoelectricity
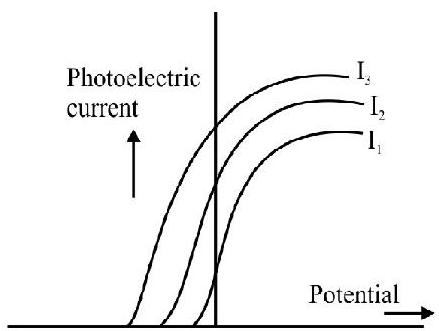
8. Incident radiation, of a given frequency, (but variable intenstiy) is incident on a given photosensitive surface. The graph, that represents the variation of the photoelectric current, with the potential (applied across the photoelectric tube), is the graph numbered as graph.
(1)
(2)
(3)
(4)
(A)
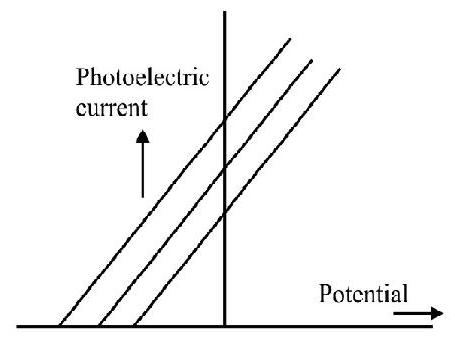
(C)
(B)
(D)
Show Answer
Correct answer: (2)
Solution:
Option (2) is correct graph. We know that stopping potential is inpendent of intensity of the incident radiation.
Difficult
Photoelectricity
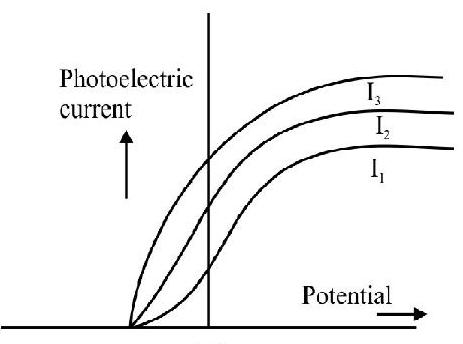
9. Photoelectric effect experiment are performed using three different metal plates
(1)
(2)
(3)
(4)
(A)
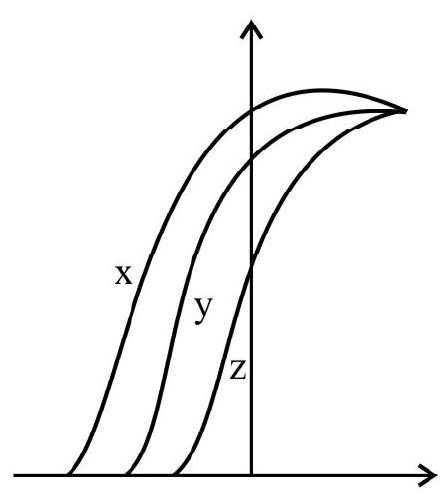
(C)
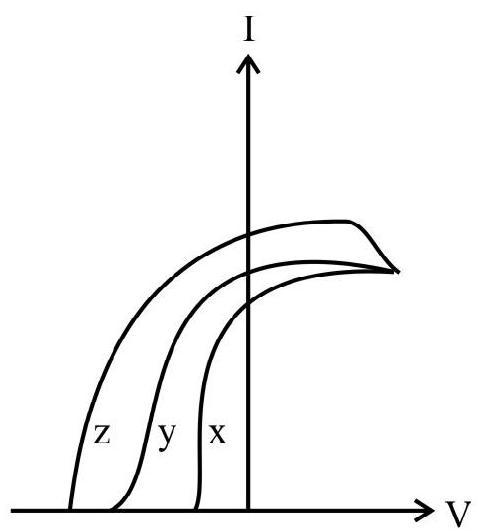
(B)
(D)
Show Answer
Correct answer: (4)
Solution:
Thus it is clear
Also work function of
Hence option (4) is correct.
Average
Photoelectricity
10. Which one of the following statement is not a part of the quantum theory of light?
(1) There is a minimum frequency of light below which no photoelectrons are emitted
(2) The maximum kinetic energy of photoelectron depends only on the frequency of light and not on the intensity
(3) Photoemission occurs when light of appropriate frequency but minimum intensity is incident
(4) The charge of photoelectrons is quantized
Show Answer
Correct answer: (4)
Solution:
Option (1),(2) and (3) are experimentally observed facts of the phenomenon of photoelectricity and are explained by Einstein’s theory of the photoelectricity. Option (4) refers to quantization of charge in general and is not a part of Einstein’s theory of photoelectric emission.
Hence option (4) is correct.
Difficult
Photoelectricity
11. The graph between stopping potential versus frequency for a photoemitter is shwon in figure. Then
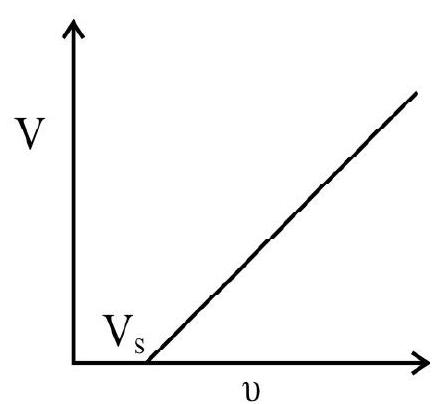
(1) The slope of the graph enable us to determine the work function of the emitter
(2) The slope of the graph is equal to Planck constant
(3) The product of the slope of the graph and electronic change is equal to Planck’s constant ’
(4) The slope of the graph enables us to determine threshold wavelength of the emitter
Show Answer
Correct answer: (3)
Solution:
According to Einstein’s photoelectric equation we have
If
Differenciating we get e
or
But
Hence
Hence option (2) is correct.
Average
Photoelectricity
12. Light of two different frequencies whose photons have energies
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (2)
Solution:
We have
Thus
or
This option (2) is correct anser.
Average
Photoelectricity
13. The work function for sodium is 2.0 volt. Then the longest wavelength of light that will eject photoelectrons from the surface is
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (1)
Solution:
The work function
Hence
or
Hence option (1) is correct.
Average
Photoelectricity
14. A photo electric cell using caesium as the sensitive element is illuminated with light of wavelength of
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (3)
Solution:
From Einstein’s photoelectric equation we have
or
Alternately;
The energy of the photon in
Hence option (3) is correct.
Average
Photoelectricity
15. The maximum kinetic energy of photoelectron emitted from a surface when photons of energy
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (2)
Solution:
Stopping potential is defined as the negative potential applied to a photoemitting surface as the negative potential which will stop the emission of the electrons from the surface even with maximum kinetic energy. Therefore stopping potential will be
Hence option (2) is correct.
Difficult
Photoelectricity
16. The energy of photoelectrons emitted from a metal surface is
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (1)
Solution:
We are given the energy of emitted photoelectrons
Hence
Also
or
Solving we get
Hence option (1) is correct
Difficult
Photoelectricity
17. The minimum light intensity that can be percieved by the eye is about
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (1)
Solution:
Let
or
Hence option (1) is correct.
Difficult
Photoelectricity
18. When a piece of metal is illuminated by monochoromatic light of wavelength
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (2)
Solution:
From Einstein’s photoelectric equation we have
From above equations
Hence
Hence option (1) is correct.
Difficult
Photoelectricity
19. The retarding potential for a given photoelectric emitter is
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (2)
Solution:
From Einstein’s photoelectric equation we
Hence option (2) is correct.
Difficult
Photoelectricity
20. The threshold wavelength of metallic silver is
(1)
(2)
(3)
(4)
Show Answer
Solution:
Now
Hence option (1) is correct.
Very Difficult
Photoelectricity
21. A beam of light has three wavelenghs
(1)
(2)
(3)
(4)
Show Answer
Solution:
The threshold wavelength will be given by
Hence light of wavelength
is
Power incident on the surface due to each wavelength is
Energy of one photon of
Power incident on
or
Energy of one photon of
Power incident of
The number electrons emitted by
or
Hence total number of
Hence option (1) is correct.
Difficult
Photoelectricity
22. The electric field associated with a light wave is given by
(1) 0.1 volt
(2) 1 volt
(3) 11 volt
(4) 1.1 volt
Show Answer
Correct answer: (2)
Solution:
The equation of a harmonic wave is given by
The electric field associated with incident light is given by
Comparing the two we get
Further
or
Hence option(2) is correct
Difficult
Photoelectricity
23. The work function of potassium is
(1)
(2)
(3) wavelengths longer than
(4) wavelengths shorter than
Show Answer
Correct answer: (4)
Solution:
The Einstein’s photoelectric equation is given by
When the electrons are just liberated
Also
Hence wavelengths shorter than
Difficult
Photoelectricity
24. A 100 watt light bulb giving monochromatic light of wavelength
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (1)
Solution:
Energy of each photon
Energy of light emitted by the bulb per second
Number of photons reaching the surface per second
Hence option(1) is correct
Difficult
Photoelectricity
25. An image of the sun is formed on the metal surface of a photocell and it produces a photoelectric current
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (4)
Solution:
The number of photoelectrons emitted from a metal surface is directly proportional to the intensity of light. When we replace the original lens with a lens of half the focal length but diameter of the lens remaining unchnaged, the intensity of light incident on metal surface remain unchanged and hence the value of the current will remain unchanged.
Hence option (4) is the correct answer.
Difficult
Photoelectricity
26. A caesium cell with a steady potential difference of 90 volt across it, is illuminated by a small light placed one meter away. When the same light is placed to two meter away, the electrons crossing the photo cell, will
(1) each carry one quarter of their previous energy
(2) each carry one quarter of their previous momentum
(3) are half as numerous
(4) one quarter as numerous
Show Answer
Correct answer: (4)
Solution:
Since the intensity of light varies inversely as the square of the distance; increasing the distance twice of the previous value the intensity of light incident on photocell will be reduced by a factor of 4 . Now the number of electrons emitted from a photo surface is directly proportional to the intensity of light, the number of photo electrons emitted will decrease by a factor of 4 . Hence they will be one quarter as numerous.
Hence option (4) is the correct answer.
Difficult
Photoelectricity
27. A monochromatic light of wavelength
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (3)
Solution:
The metallic sphere becomes positive charged as the electron are emitted from it (sphere is isolated). Hence the electrons are subjected to an extra force. If the potential of sphere is raised to
The charge on the sphere which will raise the sphere potential to
Hence the number of electrons emitted will be
or
Hence option (3) is the correct answer.
Average
deBroglie Waves
28. An electron accelerated by a p.d of
(1) remain unchanged
(2) becomes 4 times
(3) become double
(4) become halved
Show Answer
Correct answer: (4)
Solution:
When an electron is accelerated through a potential
Thus
Hence whenever
Hence option (4) is the correct answer.
Average
deBroglie Waves
29. Relative to the original photon the photon that emerges after a collision with an electron has
(1) more energy
(2) more momentum
(3) higher frequency
(4) longer wavelength
Show Answer
Correct answer: (4)
Solution:
When the photon collides with an electron it share the energy with electron according to laws of conservation of energy and momentum. Hence its energy and verocity consequently will decrease and therefore in accordance with relation
Hence option (4) is correct.
Average
deBroglie Waves
30. Which of the following graphs, represents the correct variation of the deBroglie wavelength with potential through which the electron is accelerated from rest?
(1)
(2)
(3)
(4)
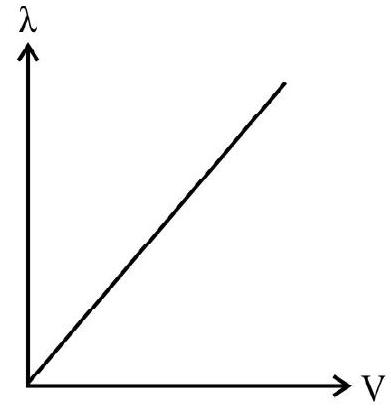
(A)
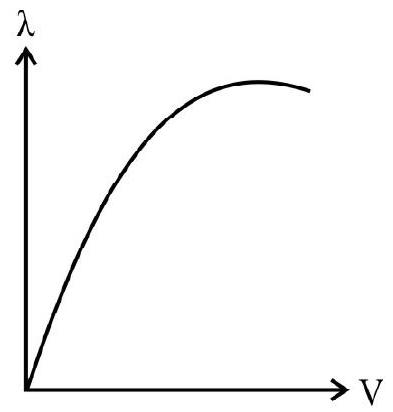
(B)
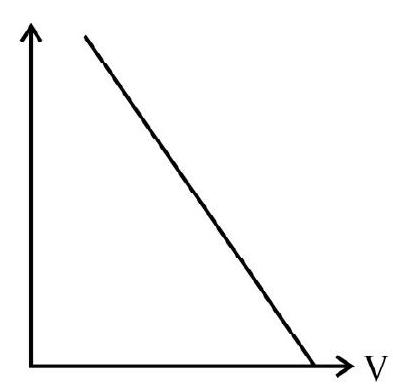
(C)
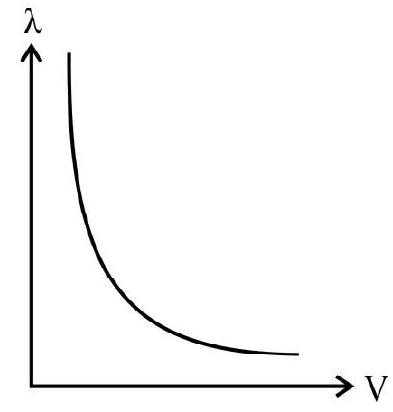
(D)
Show Answer
Correct answer: (4)
Solution:
We know that
Thus variation of
Hence option (4) is correct.
Average
deBroglie Waves
31. An electron, proton and an alpha particle all have the same kinetic energy. The deBroglie wavelength is
(1) shortest for the electron
(2) shortest for the proton
(3) shortest for the alpha particle
(4) are in the ratio of masses of electron proton and alpha particle
Show Answer
Correct answer: (3)
Solution:
The kinetic energy of a particle is
where
deBroglie wavelength
So that we can conclude that
From the electron, proton and alpha particle, the alpha particle is the heaviest. Hence it will have the shortest deBroglie wavelength.
Hence option (3) is correct.
Average
deBroglie Waves
32. A particle is moving three time as fast as an electron. The ratio of the deBroglie wavelength of the particle to that of the electron is
(1) decutron
(2) a particle
(3) a protonor a neutron
(4) particle cannot be identified from the given data
Show Answer
Correct answer: (3)
Solution:
Let
This could be the mass of a proton or a neutron.
Hence option (3) is correct.
Difficult
deBroglie Waves
33. The energy of a proton is equal to the kinetic energy of a photon. The energy of the photon
is E. Let
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (4)
Solution:
We will have
or
Hence option (4) is correct.
Difficult
deBroglie Waves
34. An electron of mass
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (4)
Solution:
When an electrons is accelerated through a electric field we have
and the acceleration of the electron is given by
Hence the velocity of the electron after it has remained in the field for a time
The wavelength of the deBroglie wave associated with this electron is
Hence option (4) is correct.
Difficult
deBroglie Waves
35. Which of the following statements is correct? (you are given the value of Boltzman’s constant)
(1) The deBroglie wavelength of thermal neutrons
(2) The deBroglie wavelength of thermal neutrons is much larger than the distance between the atoms in a crystal
(3) The deBroglie wavelength of thermal neutrons is much smaller than the distance between the atoms in a crystal
(4) A beam of thermal neutrons cannot be used for investigating lattice structure by diffraction techniques
Show Answer
Correct answer: (1)
Solution:
The wavelength of neutrons of mass
Also
Also
Now
Putting the values of the constants and solving we get
Thus the wavelength of thermal neutrons is of the order of few angstron units which is same as the order of distance beween the atoms in a crystal.
Hence option (1) is correct.
Difficult
deBroglie Waves
36. The deBroglie wavelength of electron in ground state of hydrogen atom is (given that the radius ofthe first orbit of hydrogen atom’s
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (2)
Solution:
Since the electron is in ground state which implies that electron is in the first orbit of hydrogen atom with radius
Hence option (2) is correct.
Average
Photoelectricity
37. The kinetic energy of electron is
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (2)
Solution:
We have
or
or
Hence option (2) is correct.
Difficult
deBroglie Waves
38. An electron is moving will an initial velocity
(1) remains constant
(2) increases as the electron moves round a circular path
(3) decreases as the electron moves round a circular path
(4) increases and decreases periodically
Show Answer
Correct answer: (1)
Solution:
When a particle moving in a direction perpendicular to the direction of the applied field the force acting on the electron is perpendicular to both the direction of moving particle and the field direction
Hence option (1) is correct.
Difficult
deBroglie Waves
39. A particle moves in a closed orbit across the origin due to a force which is directed towards the origin. The deBroglie wavelengh of the particle varies cyclically between two values
(1) The particle could be moving in a circular path with origin as centre
(2) The particle could be moving in an elliptical orbit with origin as it focus
(3) When the particle is having deBroglie wavelength to
(4) The particle is at the same distance from the origin whether having deBroglie length
Show Answer
Correct answer: (2)
Solution:
Since it is given that
Since
It can also be seen from the figure that
Hence option (2) is correct.
Difficult
deBroglie Waves
40. Which of the following statement is correct?
(1) The wave properties of matter can mainfest themselves with a moving particle of any size and mass
(2) The matter waves hypothesis contradicts the theory of relativily
(3) We associate a single well defined wavelength with a moving particle localized in a well defined region of space
(4) It is deBroglie wave group associated with a moving particle travelling with the same velocity as the moving particle
Show Answer
Correct answer: (4)
Solution:
The deBroglie wave associated with a moving particle cannot be a monochromatic wave as a wave train is infinite in extent and cannot be locarized in a well defined region of space. However a particle is well localized in space. According to detailed analysis, we associate a wave packet formed by the superposition of a number of waves situated on and around the central wavelength. The velocity of the component monochromatic waves making up the wave packet is known as PHASE velocity whereas the speed with which a wave packet of deBroglie waves travel is known as group velocity. It can be shown that it is the group velocity of wave packet with which a moving particle travel.
Hence option (4) is correct.
Average
Photoelectricity
41. The work function for aluminium surface is
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (1)
Solution:
The cut off wavelength is given by
or
Hence option (1) is correct.
Average
Photoelectricity
42. A metal surface ejects electrons when green light is incident on it but no emission take place when yellow light is incident onit. The electrons will be emitted when the surface has
(1) Red light
(2) Blue light
(3) Heat rays
(4) Infrared light
Show Answer
Correct answer: (2)
Solution:
Green light ejects electrons but yellow does not. The only option out of given four only blue light can cause emission (VIGB YOUR).
Hence option (2) is correct.
Average
Photoelectricity
43. X-rays of frequency
(1) The value of stopping potential is same for both sodium and aluminium surfaces
(2) The value of stopping potential is more for aluminium than for sodium
(3) The stopping potential is more for sodium than for aluminium
(4) The stopping potential also depends on the intensity of light used which is not specified in the question so, one cannot say which of the two metals will have a greater value for stopping potential
Show Answer
Correct answer: (3)
Solution:
From the relation
it is clear that the value of stopping potential for a given metal depend upon
Now
The metal surface having lower value of work function will have a larger value of stopping potential.
Hence option (3) is correct.
Average
Photoelectricity
44. The photoelectrons emitted from a metal surface are such that
(1) They all have same energy
(2) Their energies varies from zero to infinity
(3) Their energies vary from zero to a finite maximum
(4) Their energies vary randomly
Show Answer
Correct answer: (3)
Solution:
When incident photon liberates a electron not from the surface but inside the emitter the photoelectric equation becomes
where
Hence the option (3) is correct.
Average
deBroglie Waves
45. deBroglie wavelength of a photon is
(1)
(2)
(3)
(4) 2 Joules
Show Answer
Correct answer: (3)
Solution:
From deBroglie relation we have
Now K.E
Thus K.E
Subsituting the values of
K.
Hence option (3) is correct.
Difficult
deBroglie Waves
46. X rays of wavelength
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (3)
Solution:
Frequency of incident
Energy of emitted electron
Now
Hence option(3) is correct
Difficult
deBroglie Waves
47. The deBroglie wavelength at which the relevastic correction becomes important for an electron is
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (4)
Solution:
Since
when
when
when
when
As in option (4), we find that when a electron has a wavelength of
Hence option(4) is correct
Average
Davisson and Germer Experiment
48. If a strong diffraction peak is observed when electrons are incident at an angle
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (1)
Solution:
The option (1) is based on Bragg’s law of diffraction from a crystal planes on the basis which
Hence option (1) is correct
Difficult
Davisson and Germer Experiment
49. The first order Bragg maximum of electron diffraction in a nickel crystal
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (1)
Solution:
From Bragg’s law we have
or
or
Also from deBroglie’s relation
Hence option (1) is correct
Difficult
Davisson and Germer Experiment
50. In a Davisson and Germer experiment it was observed that when a beam of
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (2)
Solution:
From Bragg’s relation
From deBroglie relation
Hence option (2) is correct.
Difficult
Threshoold Frequency
51. For a constant frequency of the incident light, the graph between frequency
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (2)
Solution:
From the graph for metal
For metal
Hence option(2) is correct
Average
Einstein’s Photoelectric Equation
52. Lights, of frequencies
(1) 1 and
(2) 1 and
(3)
(4)
Show Answer
Correct answer: (1)
Solution:
Let
Comparing this with the standard equation,
we find that the slope, and intercept, of the given graph, are
1 and
Hence option(1) is correct
Difficult
Combination of Concepts of Particle Nature of Radiation and Wave Nature of Particles
53. The photons, of light of wavelength
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (4)
Solution:
Momentum of photon
Work function
Hence option (4) is correct
Average
Using de-Broglie Reltion for Photons
54. A beam of photons of light of wavelength,
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (3)
Solution:
The momentum of each photon,
Its change in momentum, after getting reflected back, is
Rate of change of momentum
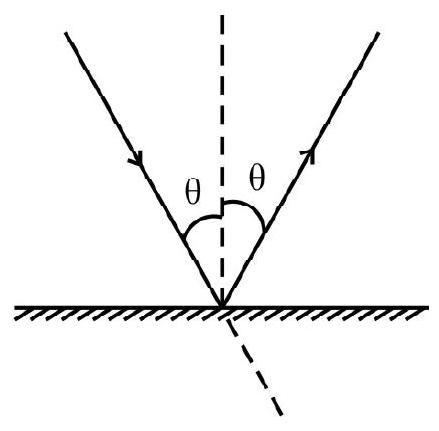
Hence option (3) is correct
Average
Einstein’s Photoelectric Equation
55. When light, of wave length
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (3)
Solution:
We have, as per Einstein’s photoelectric equation:
Also
Since
Hence option (3) is correct
Average
Einestein’s Photoelectric Equation
56. When light, of frequency
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (2)
Solution:
We have, as per Einstein’s photoelectric equation,
Hence option(2) is correct
Average
Graph between ‘v’ and ‘V’
57. The intercepts, on the ’
(1) (The threshold frequency) and
(2) (The threshold frequency) and (’
(3) (h times the threshold frequency) and (e) times the work function), of the given photosensitive surface
(4)
Show Answer
Correct answer: (1)
Solution:
We have, from Einstein’s photoelectric equation,
Intercept
The corresponding frequency, therefore, equals
Intercept
Hence option(1) is correct
Difficult
‘Stopping Potential’
58. The path of an electron beam, moving in a uniform (and normal) magnetic field of
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (2)
Solution:
For the electron beam, moving in a normal magnetic field, we have
Work function
Hence option(2) is correct
Difficult
deBroglie Reltion + ‘Stopping Potential’
59. The wave length, of a monochromatic beam of light, is
The slope of the graph, between
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (1)
Solution:
The deBroglie wavelength,
Hence option(1) is correct
Average
deBrogli Wavelength
60. The short wavelength limit of the
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (2)
Solution:
The energy of the accelerated beam of electrons
The corresponding deBroglie wavelength is
Hence option(2) is correct
Difficult
Photoelectric Emission and deBroglie Wavelength
61. A monochromatic beam of light, of wavelength
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (2)
Solution:
Energy of a photon of the incident radiation
Work function
Let
Hence option(2) is correct
Difficult
deBroglie Wavelength; Photon Energy
62. The resolving power, of an electron microscope (using on electron beam acelerated through a potential
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (4)
Solution:
The (deBrogli) wavelength, associated with the electron beam, in the electron microscope, is
Hence option(4) is correct
Difficult
deBroglie Wavelength and Energy of a Photon
63. The K.E. (K), of the electrons, having an associated deBroglie wavelength,
(1)
(2)
(3)
(4)

Show Answer
Correct answer: (4)
Solution:
The deBroglie wavelength
Energy (E) of the a photon of e.m. radiation, of wavelength, is given by
Since
The graph (D), is the only graph that correctly shows this relation between
Hence option(4) is correct
Average
Threshold Wavelength, deBroglie Relation
64. The threshold wavelength of a given photosensitive suface is
If
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (3)
Solution:
Energy of a photon of the incident light
Work function
The deBroglie wavelength, associated with an electron, accelerated through a potential
Hence option (3) is correct
Average
deBroglie Relation + Photon Energy Relation
65. The deBroglie wavelength, associated with an electron, and the wavelength, associated with a
(1) 0.82
(2) 1.64
(3) 8.2
(4) 16.4
Show Answer
Correct answer: (1)
Solution:
For the electron,
For the
Hence option(3) is correct
Average
deBroglie Relation + Photon Energy Relation
66. It is known that the average kinetic energy of a free electron, in a metal, is
(1)
(2)
(3)
(4)

Show Answer
Correct answer: (3)
Solution:
The (average) deBroglie wavelength, associated with the free electrons, in a metal, at a temperature
The wavelength,
Since
This gives
or
It is graph
Hence option (3) is correct
Difficult
Photon Energy and Power of the Source
67. A monochromatic point source, of power
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (2)
Solution:
The energy of each photon
The aperture is at a distance
The aperture area is
Hence option(2) is correct
Average Einstein’s Photoelectrons Equation
68. When monochromatic light radiations, of wavelength
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (1)
Solution:
From Einstein’s photoelectric equation, we have
Hence option(1) is correct
Average
Einstein’s Photoelectric Euqation
69. A uniform (normal) magnetic field, B, bends the path of the most energatic photoelectrons, (emitted from a given photosensitive surface due to the action of monochromatic radiations of wave length
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (3)
Solution:
Let
Now
or
Hence option(3) is correct
Difficult
No. of Photons Emitted by a Source of a Given Power
70. A light source, of power
A circular lamina, of radius
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (1)
Solution:
The power, associtaed with the radiation of wavelength
Energy of one photon
At a distance d, the number of these photons, per unit area, is
In a time of t seconds, the number of emitted photoelectrons would be nt. The charges carried by them, equals nte. Hence
Hence option(1) is correct
Average
Threhold Frequency
71. The ‘stopping potential’, needed to reduce the ‘photocurrent’ to zero, in a given set up, is 1.99 V. The given ‘set-up’, has a photosensitive surface being irradiated by a monochromatic radiation of wavelength
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (2)
Solution:
Energy of a photon, of the incident radiation,
Let
Hence option(2) is correct
Difficult
Threshold Wavelength; deBroglie Relation
72. A given photosensitive surface is being irradiated by a monochromatic radiation of wavelength
The deBroglie wavelength, associated with a beam of electrons, accelerated through a potential
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (4)
Solution:
Let
If
Also, the deBroglie wavelength,
Since
or
or
Hence option(4) is correct
Average
Einstein’s Photoelectric Equation
73. When monochromatic radiations, of frequencies
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (1)
Solution:
From Einstein’s photoelectric equation, we get
where
We then have
This gives
Hence option(1) is correct
Average
deBroglie Relation
74. The (average) kinetic energy of the free electrons ( mass
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (3)
Solution:
The deBroglie wavelength,
Hence option(3) is correct
Difficult
deBroglie Relation; Einstein’s Photoelectric Equation
75. The deBroglie wavelength, associated with electrons, accelerated through a potential
The relation, between
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (1)
Solution:
We can write
The work function, of the photosensitive surface = energy of a photon of its ’threshold wavelength’ radiation
Energy of a photon of the incident radiation
This is the required relation between
Hence option(1) is correct
Average
deBroglie Wavelength
76. It may be assumed that the average kinetic energy, of a free particle, at a temperature
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (1)
Solution:
For the electron, we have
For the
Since
or
Again, we have, for the electron
and for the
Since
or
Comparing the two ratios, we get
or
Hence option(1) is correct
Average
Graphs Related to Photoelectric Effect
77. For a constant intensity of the incident radiation, the graphs between the frequency
Let the intercepts, of these graphs, on the ‘potential’ and the ‘frequency’ axes, be denoted by
(1)
(2)
(3)
(4)

Show Answer
Correct answer: (2)
Solution:
As per Einstein’s photoelectric equation, we have
Similarly, the intercept on the
or
or
The required graph is therefore, a straight line passing through the origin and having a negative slope (slope
These requirement are met only by the graph labelled as graph B.
Hence option(2) is correct
Difficult
deBroglie Wavelength
78. An
The deBroglie wavelengths, associated with this
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (2)
Solution:
As the [positively charged,
The deBroglie wavelength, associated with the
We thus have
This is the required relation.
Hence option (2) is correct.
Average
deBroglie Relation; Photon Energy Relation
79. The kinetic energy, of an electron, having an associated deBroglie wavelength
(1)
(2)
(3)
(4)

Show Answer
Correct answer: (3)
Solution:
For the electron, having a kinetic energy K, we have, as per deBroglie relation,
Also, photon energy
We this have
The graph, between
Hence option (3) is correct.
Average
Relating deBroglie Wavelength with Photon Wavelengths
80. Three charged particles, having charges
(1)
(2)
(3)
(4)
Show Answer
Correct answer: (3)
Solution:
The kinetic energy, acquired by a particle of charge q, when accelerated (from rest), through a potential V, is
Also deBroglie wavelength,
The wavelength
As per the statement given, we have
Similarly
Hence option (3) is correct.










