UNIT - 2 Solutions
A solution is defined as a homogeneous mixture of two or more constituents (chemical species), whose composition can be varied within certain limits. These constituents cannot be separated by filtration, sedimentation or centrifugation. A binary solution has two constituents, ternary three, quaternary four. The solutions may be gaseous, liquid or solid. The physical state of a solution is the physical state of solvent. Generally, the constituent present in small quantity referred to as solute and the one present in large quantities is referred to as solvent.
The amount of solute dissolved in given amount of solvent/solution is referred to as the concentration of solute. This chapter focuses on solution obtained by dissolving solid/ liquid / gas as in a liquid.
In a given solution, let solute be represented by (1) and the solvent by (2)
Methods of Expressing the Concentration of a solution
The concentration of a solution can be expressed in many ways. A few widely used terms are:
(a) Percent by volume (v/v) : The volume of solute in
(b) Percent mass by volume (w/v) : The mass of solute present in
(c) Strength : The amount of solute in grams present in
(d) Molarity (M) : The number of moles of solute per litre or per
(e) Mole fraction
(f) Molality
(g) Normality (N) : The number of gram equivalents of solute present per litre of solution.
(h) Formality (F) : The number of formula mass in gram present per litre of solution. In case, formula mass is equal to molecular mass, formality is equal to molarity.
(i) Parts per million (
Relation between various concentration terms
(i) Mole fraction and molality
(ii) Molarity and mole fraction :
Mass of solution
Molarity
Dividing both numerator and denominator by
(iii) Molarity of mixing : Let there be three samples of solution (containing same solvent and solute) with their molarity
where,
(iv) Molarity (M) and Molality (m)
Molarity means M moles of solute in
mass of solute
mass of solution
Molality
Note :
(a) Molality is the most convenient method to express concentration and is independent of temperature.
(b) Molarity is dependent on volume and hence on temperature as well.
(c) A millimolar solution is
(d) Molarity can also be expressed in terms of density (d) of solution.
Let it contains
mass of solute in
Molarity
Solved Examples:
Question 1. A solution is prepared by mixing equal volumes of solution
Show Answer
Answer:- For calculating molality of the resulting solution : Let the volume of two solutions taken be
Solution A
Mass of
Mass of
Mass of
Solution B
Mass of
Mass of
Mass of water
Total mass of
Total mass of water (solvent)
moles of
Molality
For Calculating molarity of the resulting solution:
Let the mass of each solution taken be
Solution A
Volume of
No. of moles of
Molarity of this solution ’
Solution B
Volume of
No. of moles of
Molarity of this solution
Molarity of solution obtained by mixing equal volumes of solutions
Let
The molarity of solution
Question 2. A solution (density
(i) mass percent of anhydrous sodium carbonate
(ii) mole fraction of anhydrous sodium carbonate
(iii) molalities of
(iv) molarity of the solution
Show Answer
Solution:-
Density of solution
Total mass of solution
(i) Mass percent of
Molar mass of anhydrous sodium carbonate,
(ii) No. of moles of sodium carbonate
No. of moles of
molality of
molality of
Molar mass of anhydrous
Molarity of the given solution
Molarity
PRACTICE QUESTIONS
Question 1- A molal solution is one that contains one mole of solute in
(a)
(b)
(c)
(d)
Show Answer
Answer:- aQuestion 2- In which mode of expression, the concentration of a solution remains independent of temperature?
(a) Molarity
(b) Normality
(c) Formality
(d) Molality
Show Answer
Answer:- dQuestion 3- Dissolving
(a)
(b)
(c)
(d)
Show Answer
Answer:- cQuestion 4- The molarity of a solution obtained by mixing
(a)
(b)
(c)
(d)
Show Answer
Answer:- aQuestion 5- If equal volumes of
(a)
(b)
(c)
(d)
Show Answer
Answer:- dQuestion 6- How many millilitres (
(a)
(b)
(c)
(d)
Show Answer
Answer:- bQuestion 7- The normality of
(a)
(b)
(c)
(d)
Show Answer
Answer:- cQuestion 8- Mole fraction of glucose in
(a) 0.18
(b) 0.1
(c) 0.017
(d) 0.021
Show Answer
Answer:- dQuestion 9- The molarity of pure water is
(a) 55.6
(b) 50
(c) 100
(d) 18
Show Answer
Answer:- aQuestion 10- A 5.2 molal aqueous solution of methyl alcohol
(a) 0.190
(b) 0.086
(c) 0.050
(d) 0.100
Show Answer
Answer:- bTYPES OF SOLUTIONS
Out of various types of solutions, we shall focus on solutions obtained by dissolving
(i) gas in liquid
(ii) liquid in liquid
(iii) solid in liquid
Gas in liquid : A few examples are aerated drinks, natural water, house cleaner. Factors affecting the solubility of gas in liquid
(i) Nature of the gas and liquid
(ii) Temperature
(iii) Pressure
Dissolution of a gas in a liquid is an exothermic process therefore solubility of most of the gases in liquid decreases with increase of temperature, It is because of this, the Global warming is alarming for survival of aquatic life as it is decreasing the concentration of the dissolved oxygen in water. Pressure is the most important factor that influences the solubility of gas.
Effect of pressure on the solubility of a gas in a liquid (Henry’s law)
- Mass of a gas dissolved in a given volume of liquid at constant temperature is directly proportional to the pressure of the gas in equilibrium with the liquid.
- For mixture of gases in equilibrium with a liquid, the partial pressure of each gas is directly proportional to the mole fraction of the gas in the solution.
(a)
(b) Greater the value of
(c)
Applications of Henry’s Law
(i) In production of carbonated beverages. Under high pressure, solubility of
(ii) In deep sea diving. Compressed air cylinder is used to get more oxygen in the blood but dissolved nitrogen causes a disease called decompression sickness. Hence, air diluted with helium is used.
(iii) In the function of lungs. In the lungs, partial pressure of
(iv) For climbers or people living at high altitude. Concentration of
Limitations
Henry’s law does not hold good if :
-
Pressure is very High
-
Temperature is very low
-
Gas combines chemically with solvent
Exceptions
The solubility of
Solubility of oxygen is blood is abnormally high because of the presence of haemoglobin in blood
Vapour pressure of a solution
Let some pure liquid be taken in a closed vessel, The liquid at the surface vapourises and fills the available space. After sometime, an equilibrium is established between the liquid and vapour phases. The pressure exerted by the vapours in this state is referred to as the vapour pressure of liquid.
If some solute(solid/liquid) is added to this solvent two possibilities arise:
(i) solute is volatile
(ii) solute is non-volatite
(i) Solute is volatile
The solution is taken in a container and closed with a lid. The vapour phase contains vapour of both solute and solvent. The total pressure exerted by these vapours is referred to as the vapour pressure of solution. The partial vapour pressure of a component in such type of solution is given by Raoult’s Law.
(ii) Solute is non-votalile
There is no contribution from solute in the vapour phase. The vapour pressure of such solution is equal to the partial vapour pressure of solvent molecules
Raoult’s law for volatile liquids
In a solution the vapour pressure of a component at a given temperature is equal to the mole fraction of that component in the solution multiplied by the vapour pressure of that component in the pure state.
Hence, plot of
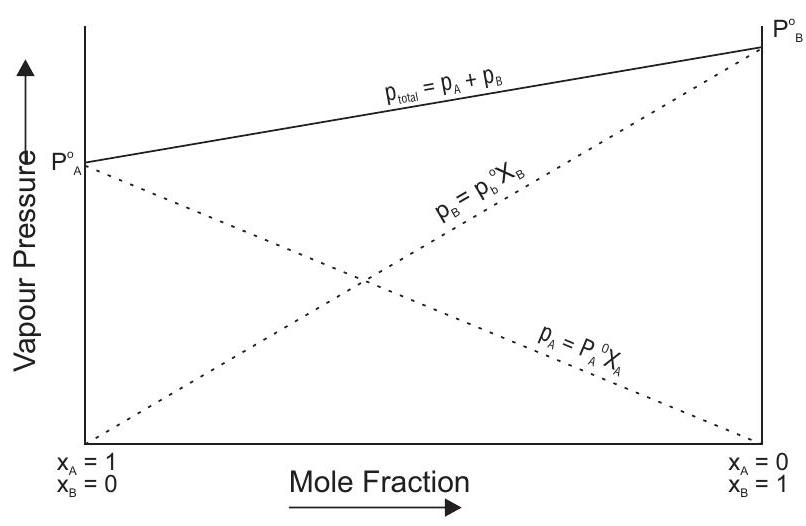
Formulation of ideal solution
Liquid pairs whose molecules are generally similar in size and structure form ideal solutions e.g. bromoethane and chloroethane
The mole fractions of the two constituents in the vapour phase are calculated as follows.
-
Raoult’s law is applicable only if the two components forming the solution have similar structure, e.g., benzene + toluene, hexane + heptane etc. Their solutions are called ideal solutions.
-
The law is not applicable if the components are not completely miscible or if the components have widely different structures.
-
Effect of adding non-volatile solute on vapour pressure of a liquid. The vapour pressure of a liquid decreases if some non-volatile solute is dissolved in it because some molecules of the solvent on the surface are replaced by the molecules of the solute.
-
Vapour pressure of solutions of solids in liquid (Raoult’s law for non-volatile solutes)
As solute is non-volatile,
V.P. of solution
or
This is Raoult’s law for non volatile solute
is known as relative lowering of vapour pressure.
Relative lowering of vapour pressure of a solution containing a non volatile solute is equal to the mole fraction of the solute in the solution.
For a dilute solution
Ideal Solutions
A solution in which each component obeys Raoult’s law under all conditions of temperatures and concentrations. In such solutions, the molecules of the liquid pair one generally similar in size and structure. Also, in such solutions :
(a)
(b)
(c) Forces between A-B are same as between A-A and B-B.
eg. Benzene + toluene, hexane + heptane, bromoethane + chloroethane
Vapour pressure diagram for ideal solutions is shown below
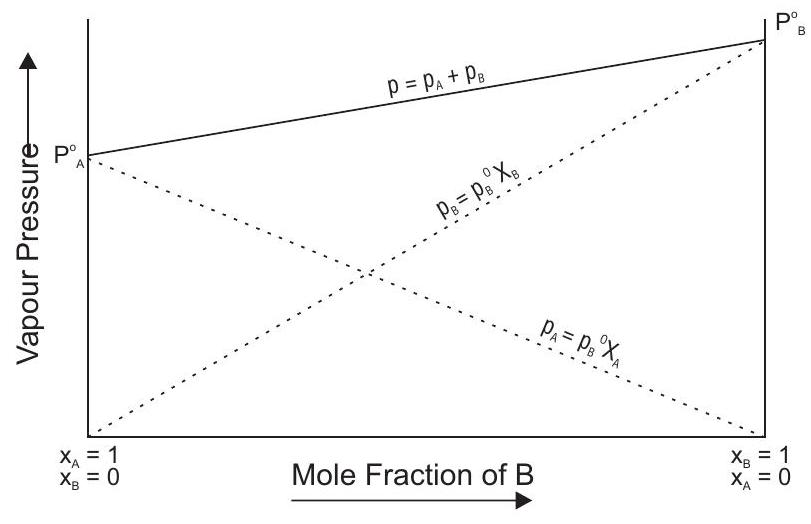
Formulation of non-ideal solution
Such solutions show either higher experimental values of vapour pressure (
Non ideal solution
| +ve deviation | -ve deviation |
|---|---|
| (a) |
|
| (b) |
|
| (c) Forces between |
Forces between |
| (d) boil at relativity low temperature e.g. methanol + water, ethanol + water | (d) boil at relatively high temperature |
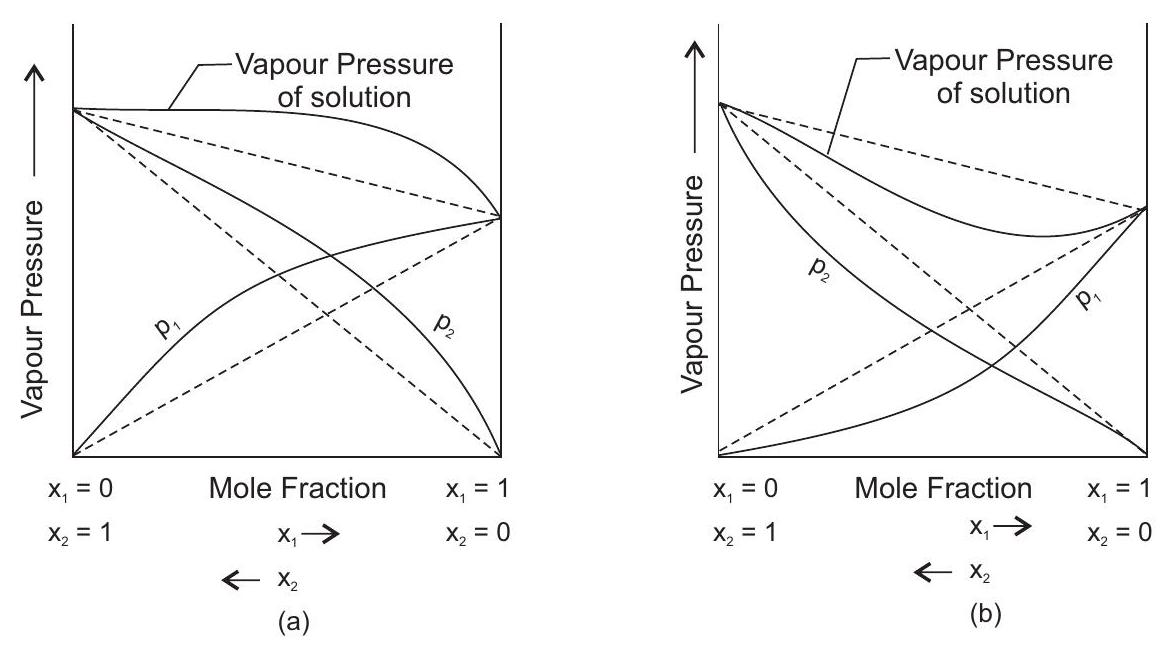
Raoult’s law is not valid when solute undergoes association or dissociation in given solvent.
Azeotropes
Liquid mixture, having the same composition, in liquid and vapour phase and boiling like a pure liquid is called a constant boiling mixture or an azeotropic mixture or an azeotrope.
Maximum Boiling Azeotropes
Solutions showing negative deviation from Raoult’s law and for which vapour pressure is minimum. The boiling point is more than either of the two components. For example, a mixture of nitric acid (b.pt.
Minimum Boiling Azeotropes
Solutions showing positive deviation from Raoult’s law and for which vapour pressure is maximum. The boiling point is less than either of the two pure components. For example, mixture of ethanol (b. pt.
Vapour pressure of liquid and temperature
Vapour pressure of liquid increases with temperature.

If vapour pressure of a liquid is known at a temperature, it can be calculated at another temperature using Clausius - Clapeyron equation
Colligative Properties
Properties which depend on the number of particles of solute and do not depend on the nature of solute. There are four colligative properties :
i) Relative lowering of vapour pressure
ii) Elevation in boiling point
iii) Depression in freezing point
iv) Osmotic pressure
RELATIVE LOWERING OF VAPOUR PRESSURE
Formula used (Raoult’s law for non-volatile solutes) for solid (non-volatile) solute dissolved in liquid,
Since relative lowering of vapour pressure depends on the number of moles, so it is a colligative property.
ELEVATION IN BOILING POINT
Boiling point of a liquid is the temperature at which vapour pressure of liquid becomes equal to atmospheric pressure. As the vapour pressure of solution is lower than that of solvent, its boiling point is higher.
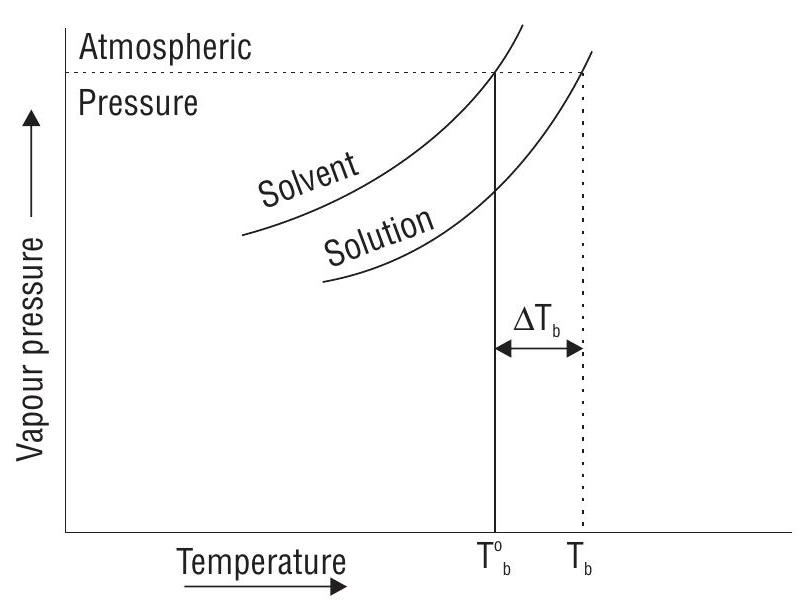
If
Ebullioscopic Constant
It is the elevation in boiling point when the molality of solution is unity.
SI unit :
Relationship between
Relationship between
DEPRESSION IN FREEZING POINT
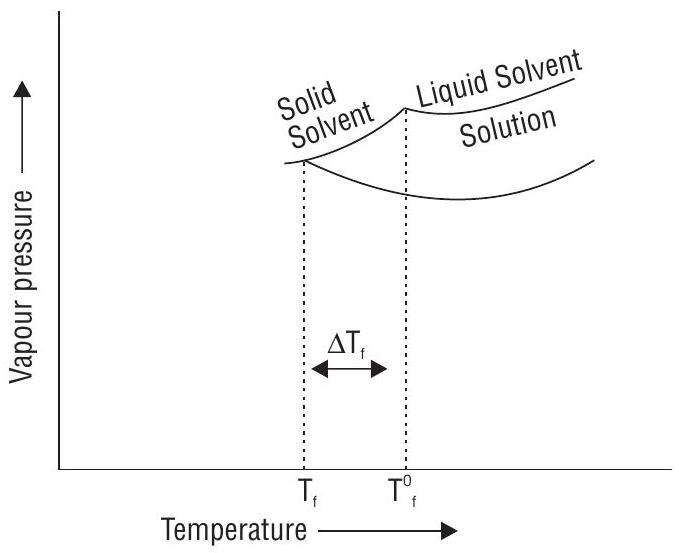
Freezing point of a substance is the temperature at which the solid and liquid forms of the substance are in equilibrium and their vapour pressures are equal. Freezing point of solution is always lower than that of the pure solvent.
Due to lower vapour pressure of the solution, solid from a solution separates out at lower temperature. The decrease is called the depression in freezing point.
Depression in freezing point is given by equation :
If
Molal Depression Constant
Depression in freezing point when molality of solution is unity. SI unit
Relationship between molal depression constant and enthalpy of fusion
Relationship between molal depression constant and latent heat of fusion
Applications of depression in freezing point
1. Ethylene glycol is used as antifreeze (causes depression in freezing point) in automobiles.
2.
OSMOSIS
Net spontaneous flow of solvent molecules from solvent to the solution or from less concentrated solution to a more concentrated solution through semipermeable menbrane.
Osmotic Pressure - Minimum excess pressure that must be applied on the solution to prevent the entry of solvent into the solution through the semipermeable membrance
Reverse 0smosis - If a pressure higher than osmotic pressure is applied on the solution the solvent will flow from the solution into the pure solvent through the semi permeable membrane. It is used in the desalination of sea water.
Isotonic solutions. Solution having the same osmotic pressure are called isotonic (or isosmotic) solutions: This is so only when they have the same molar concentration,
Knowing
-
Hypotonic and hypertonic solutions : A solution having lesser osmotic pressure (lower concentration) than the other is called hypotonic. A solution having higher osmotic pressure (higher concentration) than the other is called hypertonic.
- Example: A
- Example: A
-
Advantage of osmotic pressure method for polymers. Osmotic pressure method is the best method for determination of molar masses of polymers because for polymer solutions, observed value of any other colligative property is too low to be measured accurately. Moreover, it can be carried out at room temperature. No heating or cooling is required. Some polymers, e.g., proteins undergo a change on heating.
ASSOCIATION OR DISSOCIATION OF SOLUTE AND ABNORMAL MOLAR MASSES
-
Abnormal molar mass. When the molar mass of a substance as determined by using colligative properties, does not come out to be same as expected theoretically, it is said to show abnormal molar mass.
-
Abnormal molar mass is obtained when the substance in the solution undergoes dissociation, i.e., case of electrolytes (e.g., NaCl in water) or association (e.g., organic acids in benzene). Dissociation results in the increase in the number of particles and hence increase in the magnitude of colligative property and decrease in the molar mass (as in each formula, molar mass is inversely proportional to colligative property, e.g.,
-
Van’t Hoff factor. This factor was introduced to calculate the extent of association or dissociation
It is the ratio of the experimental value of the colligative property to the calculated value of the colligative property. It is represented by ’
As molar mass
hence,
Modified formulae for substances under going dissociation or association in the solution
- Calculation of degree of dissociation. If one molecule of a substance
Total no. of moles at equilibrium
or
e.g., in case of
- Calculation of degree of association. If
Total no. of moles at equilibrium
or
e.g., in case of benzoic acid in benzene
Comparison of Osmotic Pressure, Freezing point, Boiling point of solutions of non electrolytes and Different Electrolytes
For comparison, the value of a colligative property or molar concentrations of particles (ions or molecules) are considered. Thus,
-
As electrolytes dissociate to give ions. Solution of an electrolyte will show greater value of a colligative property than that of a non-electrolyte of same molar concentration.
-
For solutions of different electrolytes (
-
Solutions of different non-electrolytes (urea, glucose etc,) of the same molar concentration will show the same value of the colligative property.
-
Comparing solutions of the same or different non-electrolytes, greater the molar concentration, greater is the value of the colligative property.
-
In case of polymers, elevation in boiling point and depression in freezing point values are very small and difficult to be measured experimentally.
-
Osmotic pressure of mixture of two solutions of same substance having different osmotic pressure
- Osmotic pressure of mixture of two solutions when
Solved Examples
Question 3. Calculate the molar mass of the solute,
(Vapour pressure of pure benzene
Show Answer
Solution:-
The addition of solute decreases the vapour pressure of the pure liquid therefore, it is nonvolatile in nature
Vapour pressure of pure benzene
Vapour pressure of solution
on solving,
Question 4. The vapour pressure of water at
(i) vapour pressure of pure liquid
(ii) composition of two components in the vapour phase;
(iii) vapour pressure of a solution containing equal mass of the two liquids;
(iv) vapour pressure of a solution containing equal number of moles of the two liquids.
Show Answer
Solution:-
(i) No. of moles of liquid
No. of moles of liquid
on solving
(ii) Composition of
(iii) Moles of
Moles of
Total moles
(iv) Equal no. of moles
Question 5. Calculate osmotic pressure of blood at
Show Answer
Solution:-
We know,
To calculate molarity of blood, we need to just calculate
Molality of blood
The osmotic pressure of blood is
since, Colligative properties are defined for dilute solution
Question 6.
(a) A
Show Answer
Solution:-
(a) For isotonic solution
For dil solution molarity = molality
(b)
Question 7. A compound
The freezing point of the solution is lowered by
Show Answer
Solution:-
Let
Total Number of moles present in the solution
Van’t Hoff factor,
The value of ’
Molar mass of
Molality of the solution
Question 8. Arrange the following solutions in the order of increasing boiling point and freezing point, osmotic pressure.
(a)
(b)
(c)
(d)
Show Answer
Solution:-
If the density of the solution is not given, then molality and molarity are considered to be same and it is also assumed that all ionic compounds undergo
| solute | ionisation | i |
|---|---|---|
| Urea | - | 1 |
| 2 | ||
| 3 | ||
| 4 |
Greater the value of
Boiling point increases as
Freezing point decreases as
Since
Question 9. What are the structures of benzoic acid when
Show Answer
Solution:-
The molality of the solution
for benzene solution,
Similarly for acetone
The molecular mass of benzoic acid is
PRACTICE QUESTIONS
Question 1- For an ideal solution with
a).
b).
c).
d). There is no relationship between
Show Answer
Answer:- cQuestion 2- The millimoles of
a)
b)
c)
d)
Show Answer
Answer:- bQuestion 3- If two substances
a)
b)
c)
d)
Show Answer
Answer:- dQuestion 4- The mass of solute (molar mass
a)
b)
c)
d)
Show Answer
Answer:- bQuestion 5- A solution has 1:4 mole ratio of pentane to hexane. Vapour pressures of pure hydrocarbons at
a)
b)
c)
d)
Show Answer
Answer:- bQuestion 6- The elevation in boiling point, when
a)
b)
c)
d)
Show Answer
Answer:- cQuestion 7- The Henry’s law constant for the solubility of
a)
b)
c)
d)
Show Answer
Answer:- aQuestion 8- For a dilute solution containing
a)
b)
c)
d)
Show Answer
Answer:- aQuestion 9- An azeotropic solution of two liquids has boiling point lower than either of them when it
a) shows negative deviation from Raoult’s Law
b) shows no deviation from Raoult’s Law
c) shows positive deviation from Raoult’s Law
d) is saturated
Show Answer
Answer:- cQuestion 10- On mixing, heptane and octane form an ideal solution. At
a)
b)
c)
d)
Show Answer
Answer:- cQuestion 11- An aqueous solution containing
a)
b)
c)
d)
Show Answer
Answer:- dQuestion 12- An aqueous solution of glucose boils at
a)
b)
c)
d)
Show Answer
Answer:- dQuestion 13- The solubility of a gas in water at
a)
b)
c)
d)
Show Answer
Answer:- aQuestion 14- Benzene and toluene form nearly ideal solution. At
a)
b)
c)
d)
Show Answer
Answer:- aQuestion 15- The mass of glucose that should be dissolved in
a)
b)
c)
d)
Show Answer
Answer:- bQuestion 16- The relative lowering of vapour pressure of an aqueous solution containing non-volatile solute is 0.0125 . The molality of the solution is
a)
b)
c)
d)
Show Answer
Answer:- aQuestion 17- Two aqueous solutions
a) more solvent will flow from
b) more solvent will flow from
c) solvent from
d) no flow will take place
Show Answer
Answer:- aQuestion 18- When
a)
b)
c)
d)
Show Answer
Answer:- bQuestion 19- A solution has 1:4 mole ratio of pentane to hexane. The vapour pressure of the pure hydrocarbons at
a) 0.200
b)
c) 0.549
d)
Show Answer
Answer:- bPRACTICE QUESTIONS
Question 1-
a)
b)
c)
d)
Show Answer
Answer:- bQuestion 2-
a)
b)
c)
d)
Show Answer
Answer:- bQuestion 3- Which of the following
a) Potassium Sulphate
b) Sodium chloride
c) Urea
d) Glucose
Show Answer
Answer:- aQuestion 4-
a)
b)
c)
d)
Show Answer
Answer:- aQuestion 5- Freezing point of a solution containing
a)
b)
c)
d)
Show Answer
Answer:- aQuestion 6- A decimolar solution of potassium ferrocyanide is
a)
b)
c)
d)
Show Answer
Answer:- bQuestion 7- A motor vehicle radiator was filled with 8 litre of water in which 2 litre of a non-volatile solute (molar mass
a)
b)
c)
d)
Show Answer
Answer:- cQuestion 8- The solutions
a)
b)
c)
d)
Show Answer
Answer:- dQuestion 9- A solution containing
a)
b)
c)
d)
Show Answer
Answer:- dQuestion 10- A solution containing
a)
b)
c)
d)
Show Answer
Answer:- dQuestion 11-
a)
b)
c)
d)
Show Answer
Answer:- cQuestion 12- Calculate the molal depression constant of a solvent which has freezing point
a)
b)
c)
d)
Show Answer
Answer:- bQuestion 13- The freezing point of water is depressed by
a)
b)
c)
d)
Show Answer
Answer:- aQuestion 14-
a)
b)
c)
d)
Show Answer
Answer:- cQuestion 15- The freezing point depression constant of water is
a)
b)
c)
d)
Show Answer
Answer:- cQuestion 16-
a)
b)
c)
d)
Show Answer
Answer:- aQuestion 17- Van’t Hoff factors of aqueous solution of
a) F.pt:
b) V.P:
c) B.pt:
d) Osmotic pressure :
Show Answer
Answer:- cQuestion 18- If osmotic pressure of
a)
b)
c)
d)
Show Answer
Answer:- dQuestion 19- Phenol associates in benzene to certain extent to form dimer. A solution containing
a)
b)
c)
d)
Show Answer
Answer:- cQuestion 20- An aqueous solution containing
a)
b)
c) 0.835
d)










