Unit 12 Organic Chemistry Some Basic Principal And Technique (Part-B)
Tetravalency of carbon : shapes of simple molecules
Carbon forms covalent bonds with other carbon atoms and also with other elements like hydrogen, oxygen, halogens, nitrogen, sulphur etc. The formation of these covalent bonds and the tetravalence of carbon is because of its electronic configuration and the hybridization of
As we more from
Classification
All the known organic compounds have been broadly divided into the following classes on the basis of their structure.

I. Acyclic or aliphatic compounds are the ones which consist of straight chain or branched chain compounds, for example,

II. Cyclic or ring compounds are the ones which contain atoms joined in the form of a ring. These rings may be (a) Homocyclic and (b) Heterocyclic.
(a) Homocyclic compounds are those in which only carbon atoms are joined to form a ring.
They are of two types:
Alicyclic compounds
The aliphatic cyclic compounds are called alicyclic compounds. For example.

Aromatic compounds
Completely conjugated cyclic planar polyenes which obey Huckel’s rule, i.e. contain
(i) Benzenoids
Aromatic compounds and their alkyl, alkenyl and alkynyl derivatives which contain one or more benzene rings either fused or isolated in their molecules are called benzenoids. For example

These are also called arenes.
(ii) Non-benzenoids
Aromatic compounds which do not contain a benzene ring but instead contain other highly unsaturated rings are called non-benzenoids. For example,

(b) Heterocyclic compounds are those in which one or more heteroatom (e. g, 0, N, S) is also present in the ring. They are of two types:
Non-aromatic heterocyclic compounds
Heterocyclic compounds which resemble aliphatic compounds in their properties are called non-aromatic heterocyclic compounds. For example,

Aromatic heterocyclic compounds
Heterocyclic compounds which resemble benzene and other aromatic compounds in most of their properties are called aromatic heterocyclic compounds. For example,

Another classification of organic compounds is on the basis of the functional group that they may contain.
Functional Group
A functional group is an atom or a group of atoms present in a molecule which determines its chemical properties. For example,
Homologous Series
Homologous series is a family of chemically similar organic compounds, all the members of which contain the same functional group and any two adjacent (consecutive) members of which differ by a
The members of homologous series show gradation in physical properties and similarity in chemical properties.
Each homologous series can be represented by a general formula. For example,
The general formula, the functional group, the common name and IUPAC names of some important homologous series are given below :
| Chain length |
Word root |
Chain length |
Word root |
Chain length |
Word root |
|---|---|---|---|---|---|
| Math | Peot (a) | Non | |||
| Eth | |||||
| Prop (a) | |||||
| But (a) | Oct (a) |
Isomerism
Compounds having same molecular formula but different structural formula.
Types of Isomerism

I. Constitutional isomerism
Same molecular formula but different connectivity of atoms, that means atoms are bonded in a different sequence in the molecule. It is of five types.
1. Chain
2. Position
3. Functional
4. Metamerism
5. Tautomerism
6. Ring chain
1.Chain isomers
Isomers having different length of parent chain.

2.Position isomers
Isomers having different position of functional group on the same chain of carbonatoms.

3.Functional isomers
Isomers having different functional groups.
(a) Alcohols and ethers
Propanol
(b) Aldehydes, ketones and unsaturated alcohols
(c) Acids and ester
propanoic acid
(d) Cyanides and isocyanides
ethylcyanide
e. Nitro and nitrite

4.Metamerism
Isomerism in which number of carbon atoms on either side of functional group are different. Metamerism can be considered as a special case of position isomerism.

5. Tautomerism
(a) Arises due to migration of a hydrogen atom from one atom to other within same molecule with necessary rearrangement.
(b) Isomers exist in dynamic equilibrium and are readily interconvertible.
(c) A compound can show tautomerism only if it has electron withdrawing groups like-CO,
(d) Compound having quinonoid structure do not show tautomerism.

Tautomerism is further classified by naming the functional groups. For example keto-enol tautomerism

6.Ring chain isomerism
Ring-chain isomerism is shown by compounds which have different chain length and ring size but the same molecular formula. For example

Practice Questions
1. The enolic form of acetone contains
(a) 9 sigma bonds, 1 pi bond and 2 lone pairs
(b) 8 sigma bonds, 2 pi bonds and 2 lone pairs
(c) 10 sigma bonds, 1 pi bond and 1 lone pair
(d) 9 sigma bonds, 2 pi bonds and 1 lone pair
Show Answer
Answer: (a)2. Amongst the following, a pair representing tautomerism is:

Show Answer
Answer: (c)3. Number of constitutional isomers of the formula
(a) 4
(b) 8
(c) 10
(d) 6
Show Answer
Answer: (b)4. Methoxymethane and ethanol are
(a) functional isomers
(b) optical isomers
(c) position isomers
(d) chain isomers
Show Answer
Answer: (a)5. Metamers of ethyl propionate are
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)II Stereoisomerism
Isomers having same connectivity but different arrangement of atoms / groups in space.
1.Configurational isomerism
Different spatial arrangements of atoms in a molecule which are readily interconvertible without breaking and formation of bonds.
(a) Geometrical isomerism
(cis-trans isomerism) : arises because of restricted rotation around the double bond.

For cis-trans isomerism, two identical groups should not be present on same
Physical properties of geometrical isomers.
1.Melting point

Trans isomer being symmetrical fits well into the crystal lattice and greater energy is required to break the lattice.
2. Boiling point

Since cis isomer has higher dipole moment than trans isomer so it has higher boiling point.
3. Dipole moment

Cis is more polar than trans thus
Geometrical isomerism in nitrogen compounds aldoxime.

(b) Optical isomerism
Compounds that rotate plane polarized light and are non superimposable on its mirror image.
Polarimeter
An instrument to measure the angle of rotation of plane polarized light.
Laevorotatory
Optical isomers which rotate plane polarized light to left and are indicated by placing a
Dextrorotatory
Optical isomers which rotate plane polarized light to right and are indicated by placing a
Chiral carbon
Carbon atom bonded to four different atoms or groups (Asymmetric carbon)

An asymmetric or chiral compound rotates the plane of polarized light and is the simplest source of optical activity.
Enantiomers
Stereoisomers which are non superimposable mirror images. They have same physical properties except direction of rotation of plane polarized light and thus they cannot be separated by fractional distillation / crystallization. They have same chemical properties towards optically inactive reagents.

Diastereomers
Configurational isomers which are not mirror images. They have different physical and chemical properties and can be separated by physical techniques. They exist only when a compound has two or more assymetric carbon atoms.

Meso form
Compounds containing chiral carbon but superimposable on its mirror image. They are optically inactive due to presence of plane of symmetry.

Racemic mixture (or racemic modification)
When both the enantiomers are mixed together in the same ratio, the resulting mixture will have zero optical rotation, since the rotation values of the two isomers cancel each other (having the same absolute value of rotation but in opposite directions). The racemic mixture is thus optically inactive. Racemic mixture is represented by placing (
Racemisation
Process of conversion of an enantiomer into a racemic mixture.
Points to Remember
Calculation of number of optical isomers:
(a) If the molecule is not divisible into two identical halves and has
(b) If the molecule is divisible into two identical halves and has even number of chiral carbon atoms i.e.
2.Conformational isomerism
Different spatial arrangements of atoms that result from rotation about a C-C single bond in alkanes. Infinite number of spatial arrangements are thus possible. A small amount of energy is required for this rotation and thus the rotation is not completely free. Each spatial arrangement hence obtained is known as conformation or conformer or rotamer.
Conformations of ethane

Staggered conformation
Conformation with
Eclipsed conformation
Conformation of
Torsional strain
Repulsions between the bonding electrons of the substituents along the axis of rotation.
Conformations of butane


Relative stability of conformers of butane
anti > gauche or skew >eclipsed > fully eclipsed
Due to steric hinderance in gauche, anti is more stable. But sometimes gauche becomes more stable as in the example below.

Practice Questions
1. Which of the following compounds are chiral?

Show Answer
Answer: (d)2. Which of the following compounds possesses a chiral centre?

Show Answer
Answer: (b)3. Which of the following compounds will exhibit geometrical isomerism?
(a) 2-butene
(b) 2-butyne
(c) 2-butanol
(d) butanal
Show Answer
Answer: (a)4. Isomers which can be interconverted through rotation around a single bond are
(a) conformers
(b) diastereomers
(c) enantiomers
(d) positional isomers
Show Answer
Answer: (a)5. How many optically active stereoisomers are possible for butane-2,3-diol?
(a) 1
(b) 2
(c) 3
(d) 4
Show Answer
Answer: (b)6. Which of the following compounds will show geometrical isomerism.
(a) 1-phenyl-2-butene
(b) 3-phenyl-1-butene
(c) 2-phenyl-1-butene
(d) 1,1-diphenyl-1-propene
Show Answer
Answer: (a)7.

(a) partially eclipsed
(b) eclipsed
(c) gauche
(d) staggered
Show Answer
Answer: (c)8. Which among the following pairs are diastereomers?

(a) only 2
(b) only 1
(c) 1 and 2
(d) 1,2 and 4
Show Answer
Answer: (a)9. Geometrical isomerism is possible in

Show Answer
Answer: (c)10. Number of stereocentres and stereoisomers of the given compound

(a) 1 and 2
(b)
(c) 3 and 8
(d) 3 and 6
Show Answer
Answer: (d)IUPAC Nomenclature of Simple Monofunctional Organic Compounds
Organic chemistry deals with a large number of compounds. A systematic method has been developed so that each compound could be methodically named and is known as the IUPAC nomenclature (International Union of Pure and Applied Chemistry).
The IUPAC name of any organic compound essentially consists of three parts, i.e.,
(a) Word root (b) Suffix and (c) Prefix
Word root
Select the word root to denote the longest possible continuous chain of carbon atoms containing the functional group. The word roots for chains containing 1 to 12 carbon atoms are given below:
| General Formula | Functional group | Common < name | IUPAC name |
|---|---|---|---|
| Alkyl puldes | Holo | ||
| Alcohols | |||
| Eithers | |||
The extra ‘a’ given in parenthesis is used only if the suffix to be added to the word root begins with a consonant. For example,
Suffix
These are of two types:
(i) Primary suffix
A primary suffix is added to the word root to indicate whether the carbon chain is saturated or unsaturated. The three basic primary suffixes are :
| Type of carbon chain | Primary suffix | General name |
|---|---|---|
| Saturated | -ane | Alkane |
| Unsaturated | -ene | Alkene |
| Unsaturated | -yne | Alkyne |
If the carbon chain contains two, three, four or more double or triple bonds, numerical prefixes such as di, tri, tetra, etc. is added before the primary suffix. For example, diene, triyne, tetraene, etc.
(ii) Secondary suffix
A secondary suffix is then added to the word root after the primary suffix to indicate the functional group present in the organic compound. Some important secondary suffixes are :
| Class of organic compound | Functional group | Secondary suffix |
|---|---|---|
| 1. Alcohols | ol | |
| 2. Aldehydes | al | |
| 3. Ketones | one | |
| 4. Carboxylic acids | oic acid | |
| 5. Esters | oate | |
| 6. Amides | amide | |
| 7. Acid chlorides | oyl chloride | |
| 8. Nitriles | nitrile | |
| 9. Thiol | thiol | |
| 10. Amines | amine |
It may be noted that while adding the secondary suffix to the primary suffix, the terminal ’
| Organic compound | Word root | Primary suffix | Secondary suffix | IUPAC name |
|---|---|---|---|---|
| Eth | an |
0 | Ethanol | |
| Prop | ane | nitrile | Propanenitrile |
Prefix
Like suffixes, prefixes are also of two types:
(i) Primary prefix
A primary prefix is used simply to distinguish cyclic from acyclic compounds. For example, in case of carbocyclic compounds, a primary suffix cyclo is used immediately before the word root. Thus,

(ii) Secondary prefix
In IUPAC system of nomenclature, certain groups are not considered as functional groups but instead are treated as substituents. These are called secondary prefixes and are added immediately before the word root (or the primary suffix in case of carbocyclic compounds) in alphabetical order to denote the substituent groups or the side chains.
The secondary prefixes for some groups which are always treated as substituent groups (regardless of the fact whether the organic compound is monofunctional or polyfunctional) are given below :
| Subsitituent group | Secondary prefix | Substituent group | Secondary prefix |
|---|---|---|---|
| Fluoro | Methoxy | ||
| Chloro | Ethoxy | ||
| Bromo | Methyl |
| -1 | Iodo | Ethyl | |
| Nitro | n-Propyl | ||
| Diazo | Isopropyl | ||
| Alkoxy | t-Butyl or tert-Butyl |

2-Fluoro-4-methoxypentane
IUPAC Nomenclature of Branched Chain and Complex Organic Compounds
Longest continuous chain or the parent chain.
Select the longest continuous chain of carbon atoms in the molecule. This is called the longest chain or the parent chain while the rest of the carbon chains are called the side chains or substituents.
Rule for larger number of side chains
If two chains contain the same number of carbon atoms then the one containing a larger number of side chains is considered the parent chain. For example,

Parent chain contains one alkyl substituent (wrong).
Lowest locant rule.
If only one side chain is present, the carbon atoms of the parent chain are numbered in such a way that the side chain gets the lowest possible number called the locant. For example, the correct locant for the

If more than one side chains are present then numbering is done so as to give smallest possible number to all the substituents.

Alphabetical order of the simple substituents
When two or more simple substituents (i.e., unbranched alkyl groups) are present on the parent chain, each alkyl group prefixed by its locant is arranged in alphabetical order (irrespective of its locant) before the name of the parent alkane. For example:

Numbering of different substituents at equivalent positions
When two different substituents are present at equivalent positions, the numbering of the parent chain is done in such a way that the substituent which comes first in the alphabetical order (written first in the name) gets the lower number.

Numerical prefixes-Naming same substituent at different positions
When the same substituent occurs more than once on the parent chain at the same or different positions, the locants of each substituent are separated by commas and suitable numerical prefixes such as ‘di’ (for two), ’tri’ (for three), tetra (for four), etc. are attached to the name of the substituents. However, the prefixes di, tri, etc. are not considered while deciding the alphabetical order of the alkyl groups.

Naming the complex substituent (or branched substituent)
In case the substituent on the parent chain is complex (i.e., it has branched chain), it is named as a substituted alkyl group by separately numbering the carbon atoms of this substituted alkyl group with the carbon atom attached to the parent chain being counted as 1 . The name of such a substituent is always enclosed in brackets to avoid confusion with the numbers of the parent chain.
The prefix iso-and other related common ones are allowed by the IUPAC nomenclature for naming alkyl groups as long as they are not further substituted, whereas the prefixes sec- and tert-are not allowed.

If there are two chains of equal length, then the chain containing more number of side chains is selected.
Nomenclature of Unsaturated Hydrocarbons (Alkenes and Alkynes)
The parent chain must contain the multiple bond regardless of the fact whether it also denotes the longest continuous chain of carbon atoms or not. For example, in structure (I), the parent chain consists of five carbon atoms while the longest continuous chain contains six carbon atoms.

If both double and triple bonds are present, the numbering of the parent chain should always be done from that end which is nearer to the double or the triple bond, i.e., the lowest locant rule for the multiple bonds must be followed. For example,

If, however, there is a choice in numbering, the double bond is always given preference over the triple bond. For example,

According to the latest convention (1993 recommendations) while naming unsaturated hydrocarbons, the locant of the double bond or that of the triple bond is placed immediately before the suffix ’ene’ or the ‘yne’ and not before the word root as was the practice being followed earlier. For example,

If however, both double and triple bonds are present in the compound, their locants are placed immediately before their respective suffixes and the terminal

Practice Questions
1. The name of 
according to IUPAC nomenclature system is
(a) 2,3-dibromo-1, 4-dichlorobut-2-ene
(b) 1,4-dichloro-2, 3-dibromobut-2-ene
(c) dichlorodiboromobutene
(d) dichlorodibromobutane
Show Answer
Answer: (a)2. The IUPAC name of
(a) 1-bromo-2-ethoxy ethene
(b) (2-bromoethoxy) ethene
(c) 2-bromo-2-ethenylethane
(d) 2-bromoethyl ethenyl ether
Show Answer
Answer: (b)3. The IUPAC name of
(a) 2-pentyn-4-en-1-0l
(b) pent-4-en-2-yn-1-0
(c) 1-penten-3-yn-5-0l
(d) 5-hydroxy-1-penten-3-yne
Show Answer
Answer: (b)4. The IUPAC name of  is
is
(a) 3-oxo-2-heptyne
(b) hept-3-yn-4-0xone
(c) hept-4-yn-3-0ne
(d) hept-3-en-4-one
Show Answer
Answer: (c)5. The IUPAC name of
(a) 3-cyanopropyne
(b) but-3-yne-1-nitrile
(c) 2-propynyl cyanide
(d) but-1-yne-3-nitrile
Show Answer
Answer: (b)6. Indicate the wrongly named compound

Show Answer
Answer: (d)7. Which of the following compound has wrong IUPAC name?

Show Answer
Answer: (c)8. The IUPAC name of 
(a) 2-(1, 1-dimethylethyl) prop-1-en-3-0l
(b) 2-(1, 1-dimethylethyl) prop-2-en-1-0l
(c) 2-hydroxymethyl-3, 3-dimethyl-1-butene
(d) 2,2-dimethyl-3-hydroxymethylbut-3-ene
Show Answer
Answer: (b)9. IUPAC name of
(a) pent-2-en-4-yne
(b) pent-1-yn-3-ene
(c) pent-1-en-4-yne
(d) pent-2-en-5-yne
Show Answer
Answer: (c)10. IUPAC name of neopentane is
(a) 2, 2-dimethylpropane
(b) 2-methlypropane
(c) 2, 2-dimethylbutane
(d) 2-methylbutane
Show Answer
Answer: (a)11. The IUPAC name of  is
is
(a) 2-Ethyl-3-methylhex-1-en-4-yne
(b) 5-Ethyl-4-methyl-hex-2-yn-5-ene
(c) 3-Methylene-4-methylhept-5-yne
(d) 5-Methylene-5-ethyl-4-methylhept-2-yne
Show Answer
Answer: (a)12. The IUPAC name of the compound  is
is
(a) 2-lodo-3-chloro-4-pentanoic acid
(b) 4-0xo-3-chloro-2-iodo pentanoic acid
(c) 4-Carboxy-4, 3-chloro-2-butanone
(d) 3-Chloro-2-iodo-4-oxopentanoic acid
Show Answer
Answer: (d)Nomenclature of Polyfunctional Organic Compounds
Organic compounds which contain two or more functional groups are called polyfunctional compounds. Their IUPAC names are obtained as follows:
Principal functional group
If the organic compound contains two or more functional groups, one of the functional groups is selected as the principal functional group while all the remaining functional groups (also called the secondary functional groups) are treated as substituents. The following order of preference is used while selecting the principal functional group.
Amine salts > carboxylic acids > sulphonic acids > anhydrides > esters > acid chlorides > acid amides
All the remaining functional groups such as halo (fluoro, chloro, bromo, iodo), nitroso (-NO), nitro (-
The prefixes for some secondary functional groups are given below:
| Secondary functional group | Prefix | Secondary functional group | Prefix |
|---|---|---|---|
| Halo | N-Alkyl amino | ||
| Hydroxy | |||
| Sulphanyl |
Cyano | ||
| Alkoxy | Carboxy | ||
| Formyl | Alkoxycarbonyl or | ||
| Carb alkoxy | |||
| Oxo | Halocarbonyl | ||
| Amino | Carbamoyl |
The rules of IUPAC nomenclature for these compounds are,
Selecting the principal chain
Select the longest continuous chain of carbon atoms containing the principal functional group and maximum number of secondary functional groups and multiple bonds.
Numbering the principal chain
Number the principal chain in such a way that the principal functional group gets the lowest possible locant followed by double/triple bond.
Alphabetical order.
Identify the prefixes and the locants for the secondary functional groups and other substituents and place them in alphabetical order before the word root:

Organic compounds may be sometimes represented by bond-line notation.
In this notation, bonds are represented by lines and carbon atoms by line ends and intersections. It is assumed that required number of

Rules for IUPAC Nomenclature of Alicyclic Compounds
The following rules are generally followed:
The names of alicyclic compounds are obtained by adding the prefix ‘cyclo’ to the name of the corresponding straight chain hydrocarbon (alkane, alkene or alkyne).

If two or more alkyl groups or other substituent groups are present in the ring, their positions are indicated by numerals, such as

If the ring contains more or equal number of carbon atoms as the alkyl group attached to it, it is named as a derivative of cycloalkane and the alkyl group is treated as a substituent group, otherwise it is named as a derivative of alkane and the cycloalkyl group is considered as a substituent group. For example,

If, however, the side chain contains a multiple bond or a functional group, the alicyclic ring is treated as the substituent irrespective of the size of the ring. For example,

If a multiple (double or triple) bond and some other substituents are present in the ring, the numbering is done in such a way that the carbon atoms of the multiple bond get lowest locants 1 and 2 and also the substituents get the lower locants at the first point of difference. For example,

1, 5-Dimethylcyclopent-1-ene
If the ring contains a multiple bond and the side chain contains a functional group, then the ring is treated as the substituent and the compound is named as a derivative of the side chain. For example,

2-(Cyclopent-3-en-1-yl) propan-1-ol
If the ring as well as the side chain contain functional groups, the compound is named as a derivative of the side chain or the alicyclic ring depending on wheather the side chain or the ring contains, the principal functional group. For example,

3-(4-Nitrocyclohex-1-en-1-yl)prop-2-en-1-oic acid

2-(2-Hydroxypropyl) cyclohexan-1-one
If, however, the alicyclic ring and the side chain contain the same functional group, the compound is named as a derivative of the side chain or the ring according as the side chain or the ring contains higher number of carbon atoms. For example,

2-(2-Hydroxylbut-1-yl)cyclohexan-1-ol
If a compound contains an alicyclic ring directly linked to the benzene ring, it is named as a derivative of benzene, i.e. the compound having lowest state of hydrogenation. For example,

Cyclohexylbenzene
If an alicyclic ring is directly attached to a carbon containing functional group, the carbon atom of the functional group is not included in the parent name of the alicyclic system. Therefore, for such systems, the following prefixes and suffixes for the functional group are commonly used
| Functional group | Prefix | Suffix |
|---|---|---|
| Formyl | Carbaldehyde | |
| Carboxy | Carboxylic acid | |
| Halocarbonyl | Carbonyl halide | |
| Alkoxycarbonyl or | Alkyl carboxylate | |
| Carbalkoxy | ||
| Carbamoyl | Carboxamide | |
| Cyano | Carbonitrile |
For example,

Nomenclature of Aromatic Compounds
When giving IUPAC names to substituted benzene compounds, the prefix of the substituent is used before the word benzene. Also, the common names of many substituted benzene compounds are also accepted, for example,

When an aromatic compound contains two or more functional groups, it is named as a derivative of the compound with the principal functional group at position 1 . For example,

In case three or more functional groups are present they are named in alphabetical order. For example,

If all the functional groups present in the benzene ring are such which are normally treated as substituent groups, the various groups are arranged in alphabetical order with the group named first in the alphabetical order getting the lowest locant provided it does not violate the lowest locant rule for all the substituents. for example,

When a substituent is such which when taken together with the benzene ring gives a special name to the molecule, then it is named as a derivative of that molecule with the substituent at position I. for example,

When a benzene ring is attached to an aliphatic compound having a functional group, it is named as a phenyl derivative of that aliphatic compound. For example,

If, however, the benzene ring is attached to an alkane, it is named as a derivative of the bigger structural unit. For example,

However, simple phenyl substituted alkenes and alkynes are named either as derivative of benzene or the alkene / alkyne. For example,

Practice Questions
1. The IUPAC name of
(a) 4-hydroxy-1-methylpentanal b. 4-hydroxy-2-methylpent-2-en-1-al
(c) 2-hydroxy-4-methylpent-3-en-5-al
(d) 2-hydroxy-3-methylpent-2-en-5-al
Show Answer
Answer: (b)2. The IUPAC name of the compound
(a) 2-cyano-2-methyl-4-oxopentane
(b) 4-cyano-4-methyl-2-pentanone
(c) 4-cyano-4-methyl-2-oxopentane
(d) 2,2 dimethyl-4-0x0-pentanenitrile
Show Answer
Answer: (d)3. Name of the compound given below is

(a) 4-ethyl-3-methyloctane
(b) 3-methyl-4-ethyloctane
(c) 2,3-diethylheptane
(d) 5-ethyl-6-methyloctane
Show Answer
Answer: (a)4. The IUPAC name of 
(a) 4-acetylhex-5-en-1-yne
(b) 2–Methylpentan–1–ol
(c) 3-(prop-2-enyl) hex-4-yn-2-one
(d) 4 -acetylhex-2-en-6-yne
Show Answer
Answer: (b)5. The IUPAC name of
(a) 2-Ethylbutan-1-0l
(b) 2-Methylpentan-1-0l
(c) 2-Ethy|pentan-1-0l
(d) 3-Ethylbutan-1-0l
Show Answer
Answer: (a)6. The IUPAC name of

(a) 6-carboxy-2,5-dimethylpent-3-enoic acid
(b) 6-carboxy-2, 3-dimethylhept-4-enoic acid
(c) 2, 3, 6-trimethylhept-4-ene-1, 7-dioic acid
(d) 2,5,6-trimethylhept-3-ene-1, 7-dioic acid
Show Answer
Answer: (d)7. The IUPAC name of

(a) 2,3-dimethylpentanoyl chloride
(b) 3,4-dimethylpentanoyl chloride
(c) 1-chloro-1-0xo-2, 3-dimethylpentane
(d) 2-ethyl-3-methylbutanoyl chloride
Show Answer
Answer: (a)8. The IUPAC name of the given compound,

(a) 2-cyclohexylbutane
(b) 1-cyclohexyl-1-ethylethane
(c) (1-methylpropyl) cyclohexane
(d) 1-cyclohexyl-1-methylpropane
Show Answer
Answer: (c)9. The IUPAC name of the compound,
 is
is
(a) 3-cyclopentylhexane
(b) 4-cyclopentylhexane
(c) 3-hexylcyclopentane
(d) 4-hexylcyclopentane
Show Answer
Answer: (a)10. The IUPAC name of

(a) acetylcyclohexadiene
(b) 1-cyclohexa-2, 4-dienylethanone
(c) 6-cyclohexa-1, 3-dienylethanone
(d) none of these
Show Answer
Answer: (b)11. The correct decreasing order of priority for the functional groups of organic compounds in the IUPAC system of nomenclature is
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)12. The IUPAC name of 
(a) 2-formyl-2-methyl-1-propanol
(b) 2,2-dimethyl-3-hydroxypropanal
(c) 2-formyl-2-(hydroxymethyl) propane
(d) 2-(hydroxymethyl) -2-methylpropanal
Show Answer
Answer: (b)13. The IUPAC name of the compound

(a) Ethoxybutane
(b) 1-Ethoxy-2-propanone
(c) 4-Ethoxy-2-butanone
(d) 1-Ethoxy-3-butanone
Show Answer
Answer: (c)Basic Principles of Organic Chemistry-I
Bond Fission & Reactive Intermediates
Types of bond fission
1.Homolytic bond fission
If a covalent bond breaks in such a way that each atom takes away one electron of the shared pair.

Occurs in non polar bonds
Favoured by high temperature, UV radiations, presence of radical initiators such as peroxides.
2.Heterolytic bond fission
When a covalent bond breaks in such a way that both the electrons of covalent bond are taken by one of the bonded atoms.
It results in the formation of charged species i.e. carbocations and carbanions.
Occurs in polar covalent bonds.
Favoured by polar solvents.
The three important reaction intermediates generated by the bond fission are
I. Carbocations are electron deficient species and have only three shared pairs of electrons (or 6 electrons) around the carbon and thus have positive charge. They are formed by heterolytic cleavage of covalent bonds in which the leaving group takes away with it shared pair of electrons.
Carbocations can be classified into the following groups,
1. Alkyl carbocations
When positive charge is present on the alkyl carbon, carbocation is known as alkyl carbocation.
Stability of alkyl carbocations can be explained by
(a) Inductive effect
(b) Hyperconjugation
According to these two effects the stability order is as follows:

If

2.Vinyl carbocation
When positive charge is present on vinylic carbon then carbocation is known as vinyl carbocation;
This carbocation is the least stable because positive charge is present on the electronegative carbon.
3.Allyl carbocation
When positive charge is present on the allylic carbon of the allyl group, the carbocation is known as allyl carbocation.
Allyl carbocations are more stable than alkyl carbocations due to resonance.
Stability of primary, secondary and tertiary allyl carbocations can be compared by (a) Inductive effect (b) Hyperconjugation (c) Resonance.

4. Phenyl methyl carbocations
When positive charge is present on benzyl carbon, carbocation is known as phenyl methyl carbocation.
Phenyl methyl carbocations are of three types:

Stability of phenyl methyl carbocations can be explained by resonance.
Structure
 No of resonating structures 10
No of resonating structures 10 Greater the number of resonating structures, greater is the stability.
5. Cyclopropyl methyl carbocations
(i) These carbocations are very stable carbocations. They are more stable than benzyl carbocations.
(ii) Stability of cyclopropyl methyl carbocations increases with every cyclopropyl group. Thus, additional cyclopropyl group has cumulative additive effect on stability. Thus,

(iii) The special stability is a result of conjugation between the bent orbitals of the cyclopropyl ring and the vacant
Stability of different types of carbocations in decreasing order


Based on their stability carbocations undergo rearrangement as follows,
1. Hydride shift

2.alkyl shift

3. Ring expansion

It releases strain of smaller rings and increases stability. Rearrangement occurs from
Carbanion
II.Carbanion
Species bearing a negative charge on carbon and possessing eight electrons in its valence shell. These are produced by heterolytic cleavage of covalent bonds in which shared pair of electrons remain with the carbon atom.
Stability of carbanions can be explained by
1.Inductive effect
(a) +l effect

Greater the + effect, lower is the stability
(b) -l effect

Greater the -I effect, greater is the stability
2. Electronegativity of carbanionic carbon
Stability of carbanion depends on the % S character of carbanionic carbon. Greater the s character, greater is the stability.

3. Delocalisation or Resonance
Allyl and benzyl carbanions are stabilized by delocalisation of negative charge.
Greater the number of resonating structures, greater is the stability.
Stability of different types of carbanions in decreasing order
Benzyl carbanion > Allyl carbanion >
III. Free radicals
Atom or group of atoms having odd or unpaired electron. They are produced by homolytic cleavage of covalent bond.
There are seven electrons in the outermost orbit of carbon free radicals.
Owing to the presence of an odd electron, a carbon radical is paramagnetic in nature. Due to this reason free radicals are highly reactive.
Free radicals are neutral electrophiles.
Stability of free radicals can be explained by
1.Inductive effect
Since free radicals are electron deficient species, presence of electron releasing groups will have a stabilizing effect on them. And the stability order will be, Tertiary > secondary > primary >
2.Hyperconjugation
Stability of alkyl free radicals can be explained by the number of contributing structures formed due to hyperconjugation. Since the delocalization is due to the presence of
structures are formed. Thus the stability order is,

3.Resonance
The stability of allyl and benzyl free radicals can be explained by resonance.
Stability of allyl and benzyl free radicals
Stability of these radicals can be explained by delocalisation or resonance.
Structure
No. of resonating structures: 10
Allyl and benzyl radicals are more stable than alkyl radicals.
Overall Stability order
Practice Questions
1. The correct stability order for the following species is

(a)(II)
(b) (I)
(c) (II)
(d)
Show Answer
Answer: (d)2. The compound which gives the most stable carbocation by losing

Show Answer
Answer: (b)3. Which one of the following carbocations is most stable?

Show Answer
Answer: (a)4. The order of stability of the following carbocations.

(a) III
(b) I
(c) I > II
(d) II
Show Answer
Answer: (d)5. Which of the following pairs is / are correctly matched?
I. Carbocation : electrophile
II. Free radical : paramagnetic
III. Carbene : Incomplete octet
IV. Carbanion: Incomplete octet
(a) Only I
(b) I and II
(c) I, II, III and IV
(d) I, II and III
Show Answer
Answer: (d)6. Which free radical is most stable?
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)7. Which allylic carbocation is the most stable?
(a)
(b)

(d) All have same stability
Show Answer
Answer: (c)8. Consider the following carbanions :
I.
II.
III.
Correct order of stability of these carbanions in decreasing order is :
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)9. Arrange the given carbanions in decreasing order of their stability :
I.
II.
III.
IV.
(a) I
(b) II
(c) II
(d)
Show Answer
Answer: (c)10. Which of the following contains three pairs of electrons in the valence shell?
(a) Carbocations
(b) Carbanions
(c) Free radicals
(d) All of these
Show Answer
Answer: (a)11. Arrange the given carbocations in decreasing order of stability

(a)
(b) III
(c)
(d)
Show Answer
Answer: (b)12. Consider the following carbocations & give decreasing order of stability
I.
II.
III.
IV.
(a)
(b)
(c) IV
(d)
Show Answer
Answer: (d)13. Which carbocation is the most stable?

Show Answer
Answer: (b)14. Which one of the carbanions is most stable?

Show Answer
Answer: (a)15. Which one of the following carbocations is most stable due to resonance?
(a) 
(b)
(c)
(d)

Show Answer
Answer: (d)NUCLEOPHILES AND ELECTROPHILES
Reagents in organic chemistry can be broadly classified as electrophilic or nucleophilic reagents. Some of their features are,
Electrophiles
Electron loving species
May be positively charged or neutral
Positive electrophiles
Neutral electrophiles
Being electron deficient, they act as Lewis acids and accept a pair of electrons.
Attack the substrate molecule at the site of highest electron density, to form a covalent bond.
Nucleophiles
Nucleus loving species
May be negatively charged or neutral
Negative nucleophiles
Neutral necleophiles
Being electron rich, they actas Lewis bases and attack on electrophilic carbon.
Attack the substrate molecule at the site of lowest electron density.
Ambident nucleophiles
Species having two nucleophilic centres, for example
Compounds which behave as electrophile as well as nucleophile
Compound in which carbon is bonded with electronegative atom

Electron Displacements in a covalent bond
Electron displacements may take place in an organic compound, either due to the presence of an atom or group attached to it or in the presence of an attacking reagent. Such electron displacements are.
1.Inductive effect
Displacement of
permanent effect
weakens with increasing distance from the substituent
+l effect
if the substituent attached at the end of carbon chain is electron donating, effect is + leffect.
t-butyl
I effect
if the substituent attached at the end of carbon chain is electron withdrawing, effect is -1 effect.
Applications
Electron withdrawing group increases acidic strength
As the distance between electron withdrawing group and

Electron donating group decreases acidic strength

2.Resonance effect
Delocalisation of
Conjugated system
A given atom or group of atoms is said to be in conjugation with an unsaturated system if, i. It is directly linked to one of the atoms of the multiple bond through a single bond, and
ii. It has a
Type of conjugation
1.

2. Positive charge,

3. Negative charge,

4. Odd electron,

5. Lone pair,

Resonance effect is a permanent effect
Resonating structures are not real, rather real structure is a hybrid of all resonating structures. It is difficult to represent the actual resonance structure by a single structure.
Due to delocalization, the carbon - carbon bond lengths in a resonating structure become equal. And the value of a carbon - carbon bond length is in between that of a carbon carbon single bond and a carbon - carbon double bond.
Delocalization lowers the resonance energy thereby stabilizing the conjugated system.
Stability of resonating structures
1. Species having complete octet is more stable than species having incomplete octet.

2. Species in which positive charge resides on most electropositive atom and negative charge on most electronegative atom is more stable.

3. Increase in charge separation decreases the stability of a resonating structure.

Stability order - I
Steric inhibition of resonance
Most important condition for resonance to occur is that the involved atoms in resonating structures must be coplanar for maximum delocalisation. If bulky groups are present on adjacent atoms planarity of orbitals is inhibited. This is known as steric inhibition of resonance.

In dimethylaniline, the orbital having lone pair of electrons present on nitrogen atom is in the plane of benzene ring hence lone pair takes part in delocalization.
In
Steric inhibition of resonance has profound effect on
1. Physical properties
2. Acidity and basicity
3. Reactivity of organic compounds
Of the two compounds given above (b) is a stronger base than (a) because the steric inhibition of resonance in (b) prevents the lone pair to take part in delocalization and thus increases the availability of lone pair on nitrogen.
Another example is of 2, 4, 6-trinitro-N, N-dimethylaniline which is a much stronger base than 2, 4, 6-trinitroaniline. In 2, 4, 6-trinitroaniline,
In nitrobenzene (I), bond length between carbon-nitrogen is in between single and double bond due to resonance.
In compound (II) bond length between carbon-nitrogen is only of single bond due to inhibition of resonance.

Groups in conjugation with benzene ring can be classified as :
+R group
Groups having - ve charge or at least one lone pair of electrons donate electrons to the benzene ring or any other conjugated system by resonance effect eg.


-R group
Groups having +ve charge on key atom when bonded with electronegative atom by multiple bond, withdraw electrons from the benzene ring or a conjugated system by resonance effect

Resonance effect operates only at ortho and para positions.
3.Hyperconjugation effect
Hyperconjugation effect is the phenomenon when
Structural requirement for hyperconjugation
Compound should have atleast one
Hybridisation of
Types of hyperconjugation
1.

2.

3.

Hyperconjugative structures

Since there is no bond between
Applications of hyperconjugation
This effect has been able to explain a number of otherwise unexplained facts,
1.Stability of alkenes
Greater the number of alkyl groups attached to the doubly bonded carbon atoms, greater is the stability of alkene. In other words, more substituted alkenes are more stable

Greater the number of
2. Directive influence of alkyl groups

Due to hyperconjugation, electron density at 0 and
3. Carbon-Carbon double bond length in alkenes

Because of hyperconjugation,
4. Stability of alkyl carbocations and alkyl free radicals
Stability

5. Reversal of inductive effect order of alkyl groups
When an alkyl group is attached to an unsaturated system such as a double bond or benzene ring, order of inductive effect is reversed

This is because greater the
Applications of conjugation and resonance
1.Aromatic character of compounds
According to Huckel’s rule, a compound is aromatic if it is
Cyclic
Planar
Conjugated
Has

2. Antiaromatic compounds
Planar conjugated cyclic systems containing

3. Non aromatic systems
Some antiaromatic compounds adopt non planar geometries to become stable. They have

cyclooctatetraene (tub shaped)
Types of Organic Reactions
Organic reactions are broadly divided into the following four categories
I) Substitution Reactions
When an atom or group of atoms from a molecule is replaced by another atom or group of atoms, it is known as a substitution reaction.
ii) Addition Reactions
When an atom or group of atoms are added to a substrate molecule, which is unsaturated, it is known as addition reaction. In this reaction there is a net gain of atoms in the product molecule.
iii) Elimination Reactions
When atoms or group of atoms are removed from a molecule resulting in the formation of multiple linkage in that molecule, it is known as elimination reaction. The loss of atoms or group of atoms may occur from the same atom or different atoms in the molecule.
iv) Rearrangement Reactions
When atoms or group of atoms within the molecule simply change their position to give the product, it is known as a rearrangement reaction. It may appear as an internal substitution reaction has occured.
Practice Questions
1. Which among the following compounds behaves both as an electrophile as well as nucleophile?
I.
II.
III. 
IV. 
(a) only 1
(b) 1 and 2
(c) only 3
(d) 2,3 and 4
Show Answer
Answer: (c)2. Consider the following alkenes:

The correct sequence of these alkenes in increasing order of their stability is :
(a) III > II > I
(b) I > II > III
(c) II > I > III
(d) II > III > I
Show Answer
Answer: (c)3. Arrange the following resonating structures of formic acid in decreasing order of stability :
(a) I > II > IV > II
(b) I > II > III > IV
(c) IV > III > II > I
(d) I > IV > II > III
Show Answer
Answer: (b)4. The correct stability order of the following resonance structures is
I.
II.
III.
IV.
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)5. Arrange the following groups in order of decreasing
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)6. Which one of the following statements is not correct for electrophile?
(a) Electron deficient species are electrophile
(b) Electrophiles are Lewis acids
(c) All +ve charged species are electrophiles
(d)
Show Answer
Answer: (c)7.
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)8. In pyridine

Number of conjugated electrons is
(a) 6
(b) 8
(c) zero
(d) 5
Show Answer
Answer: (a)9. Decreasing - I power of given groups is :
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)10. In which of the following pairs, none of the species is an electrophile?
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)11. Which of the following reactions would generate an electrophile?
I.
II.
III.
IV.
(a) I, II and IV
(b) I, II and III
(c) I, II, III and IV
(d) II, III and IV
Show Answer
Answer: (a)12. In which of the following compounds delocalisation is not possible?
(a) 1,4-pentadiene
(b) 1,3-butadiene
(c) 1,3,5-hexatriene
(d) benzene
Show Answer
Answer: (a)13. Which among the following species is an ambident nucleophile?
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)14. Which among the following is an electrophile?
(a)
(b)
(c)
(d) All of these
Show Answer
Answer: (d)15. Which one of the following species is a nucleophile:
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)16. Which one of the following compounds on gentle heating will undergo facile homolytic bond cleavage?
(a) 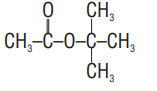
(b)
(c)
(d)
Show Answer
Answer: (d)17. Resonance is due to:
(a) delocalisation of sigma electrons
(b) migration of
(c) migration of proton
(d) delocalisation of pi electrons
Show Answer
Answer: (d)18. Hyperconjugation effect is possible in which of the following species.
(a)
(b)
(c)
(d)










