Unit 12 Organic Chemistry Some Basic Principal And Technique (Part-A)
Purification and Characterisation of organic compounds
Organic compounds, obtained from natural sources or synthesized in laboratory, are seldom pure. They are generally contaminated with impurities and therefore need purification. The principle and method of purification depend upon the nature of the substance (solid or liquid) and the type of impurity present in it.
Methods of Purification:
1.Sublimation
It involves direct conversion of a solid into the gaseous state on heating without passing through the intervening liquid state and vice versa on cooling. The process is useful to purify the compounds which sublime on heating and are associated with non volatile (solids) impurities.
Example : Camphor, naphthalene, anthracene, benzoic acid, iodine.
2.Crystallisation
This is the most commonly used technique for purification of solid compounds in laboratory. Crystals have definite geometrical shapes and are the purest form of a substance. The process by which an impure compound is converted into crystals is known as crystallisation. This method is based upon the principle that an organic compound is soluble in a suitable solvent / mixture of solvents at elevated temperature and insoluble at room temperature. For proper crytallisation choice of solvent is crucial. A suitable solvent :-
Should not react chemically with the impurities.
Should dissolve more of the substance on heating than at room temperature.
Either should not dissolve impurities or if it dissolves, it should be such an extent that impurities remain in solution (mother liquor) upon crystallisation.
Water, alcohol, chloroform, ether, benzene, acetone, ethyl acetate are commenly used solvents for crystallisation.
2.1 Fractional Crystallisation
This is the process of separation of different components of a mixture by repeated crystallisation. This method is employed to separate and purify two or more compounds having different solubilities in the some solvent.
3.Simple Distillation
It involves conversion of a liquid into vapours by heating followed by condensation of these vapours by cooling. This method is based upon the difference in boiling points of impurities and the compound to be purified. It is commonly used for the liquids, sufficiently stable at their boiling points and contain non volatile impurities.
Example: Benzene, ethanol, acetone, toluene, xylenes,
Simple distillation can also be employed for separation and purification of a mixture of two or more miscible organic liquids having sufficient difference in their boiling points. Upon distillation of such mixture, the more volatile liquid distils over first while the less volatile liquid distils over afterwards.
Example: Mixture of ether (b.p.
Mixture of hexane (b.p.
Mixture of benzene (b.p.
3.1 Fractional Distillation
This method is employed when the boiling points of liquids of a mixture are very close to each other and separation can’t be done by simple distillation. Fractional distillation is carried out in fractionating column, specially designed for the purpose to increase the cooling surface area and to provide obstructions to the ascending vapours and descending liquid. This allows repeated and successive evaporations and condensations.
Example : This method is used to separate (1) petroleum into its useful fractions such as gasoline, kerosene oil, diesel oil etc. (2) acetone (b. p.
3.2 Distillation under reduced pressure / Vacuum Distillation
This method is used for purification of high boiling liquids and the liquids which decompose at or below their boiling points. We know that a liquid boils when its vapour pressure becomes equal to the external pressure. Therefore, by reducing the external pressure acting on it, boiling point can be reduced and distillation can easily be achieved without decomposition. Water pumps / vacuum pumps are commonly used in laboratory to reduce the pressure above the liquid mixtures to be separated.
Example : Glycerol generally decomposes at its boiling point (
3.3 Steam Distillation
This is very convenient method for separation and purification of organic compounds (solid / liquid) from nonvolatile organic / inorganic impurities. Method is applicable to the compounds that are volatile in steam, insoluble in water, boil above
The impure organic compound, mixed with water is taken in a round bottom flask and steam is passed through it. When the combined vapour pressure of mixture becomes equal to the atmospheric pressure, the mixture starts boiling. At this temperature, steam mixed with the vapours of compound passes over to the condenser, gets condensed and then collected in the receiver. The distillate contains the mixture of desired compound and water which are then separated with the help of a separating funnel.
Example : Aniline, nitrobenzene, essential oils, o-nitrophenol and bromobenzene can be purified by this process.
4.Differential Extraction
This method is also known as solvent extraction. The technique is used to separate organic compounds (solid / liquid) from their aqueous solutions. In a separating funnel, the aqueous solution of organic compound is mixed with a small quantity of the suitable organic solvent. Solvent should be immiscible with water and highly miscible with the organic compound. The separating funnel is stoppered and the contents are shaken thoroughly. The organic compound, being more soluble is solvent, gets dissolved in it. The funnel is allowed to stand for some time so that the water layer separates out from the organic layer. Both the layers are separated and the process is repeated several time with the water layer in order to extract the entire / maximum amount of the organic compound. Organic compound is then recovered from the solvent through distillation / evaporation. Larger the number of extractions, greater the amount of the compound extracted. Benzene, ether, chloroform and carbon tetrachloride are some generally used solvents for extraction.
Example : Benzoic acid can be separated from its aqueous solution using benzene as a solvent.
5.Chromatography
This is the modern analytical technique, discovered by Tswett (1906) to separate the components of a mixture. The technique is based upon the principle of selective adsorption of various components of a mixture between the two phases; stationary phase and mobile phase. It is widely used, not only for separation but for purification, identification and characterization of the components present in a mixture.
Depending upon the nature of the stationary and the mobile phases, the different types of chromatographic techniques, commonly used are given in the table.
Some common types of chromatographic techniques
| S. No. | Technique | Stationary Phase | Mobile Phase | Uses |
|---|---|---|---|---|
| 1. | Adsorption or column chromatography | Solid | Liquid | Large scale separations, qualitative and quantitative analysis |
| 2. | Thin layer chromatography (TLC) | Solid | Liquid | Qualitative analysis |
| 3. | High performance liquid chromatography (HPLC) | Solid | Liquid | Qualitative and quantitative analysis |
| 4. | Gas-liquid chromatography (GLC) | Liquid (Supported on inert solid) | Gas | Qualitative & quantitative analysis |
| 5. | Partition chromatography / Paper chromatography | Liquid (supported on cellulose of paper) | Liquid | Qualitative & quantitative analysis of polar organic compounds (sugars, amino acids etc.) |
Some common terms used in chromatography
(i) Mobile phase
Phase that moves through the stationary phase. It may be liquid or a gas.
(ii) Stationary phase
It is the substance fixed in place for the chromatography. It may be solid or liquid supported on solid. For example silica layer in TLC.
(iii) Eluent
The solvent used in column chromatography is know as eluent. During elution (running) of eluent (solvent) from the column, the most weakly adsorbed component is eluted first by least polar solvent while more strongly adsorbed component is eluted later by highly polar solvents.
(iv)
It is the ratio of the distance travelled by a component to the distance travelled by the solvent front. It is a constant for a given component under a given set of conditions. Therefore, it is possible to identify the various components by determining their
Qualitative analysis of organic compounds
It is detection of various elements present in the compound.
1.Detection of carbon and hydrogen
Organic compound is heated with dry cupric oxide and produced vapours are passed through lime water or anhydrous
Compound
lime water
colourless
2.Detection of other elements
Nitrogen, sulphur & halogen are detected by Lassaigne’s test. Compound is heated with sodium metal and poured in cold & distilled water. This, after heating for sometime, gives Lassaigne’s solution or sodium extract. In Lassaigne solution, elements present in compound, get converted from their covalent to easily identifiable ionic forms
e.g.
(i) Test for nitrogen
Lassaigne extract is boiled with
Note : On heating with
(ii) Test for sulphur
(a) Sodium extract is acidified with acetic acid and lead acetate is added to it which gives black ppt of PbS and shows the presence of ’
Note: If ’
Therefore, during test of ’
Note: In excess of sodium metal, sodium thiocyanate decomposes to sodium sulphide & sodium cyanide. Therefore,
(iii) Detection of halogens
If ’
Now, sodium extract is treated with
Sod. extract
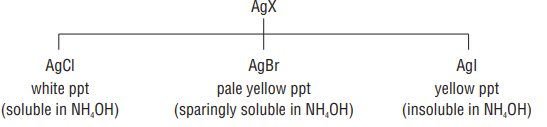
(iv) Detection of phosphorus
Organic compound is fused with sodium peroxide to convert phosphorus into sodium phosphate, which on further reaction with ammonium molybdate & conc.
Quantitative analysis of organic compounds
This is done to calculate the percentage of each element in the compound.
1.Estimation of ’
A known mass of organic compound in burnt in presence of excess of
Calculation of
For
w g CO
For
w g of
2.Estimation of ’
There are two most commenly used methods (i) Duma’s method (ii) Kjeldahl’s method.
(i) Duma’s Method: This method is applicable to all organic compounds containing nitrogen. organic compound is heated with excess of
Now, the above mixture is collected over conc.
Calculation of
(ii) Kjeldahl’s Method
Compound is heated with conc.
Organic compound
Let the mass of organic compound taken
Volume of
Volume of
Now
This is mass of ’
3.Estimation of Halogens : (Carius Method)
A known mass of organic compound is heated with fuming
Let the mass of organic compound
& mass of AgX formed = wg
Now 1 mole of
wg of AgX contains
This is mass of halogen
4.Estimation of Sulphur: (Carius method)
A known mass of compound is heated with fuming
mass of ’
5.Estimation of Phosphorus (Carius method)
When known mass of compound is heated with fuming
mass of phosphorus in wg of
6.Estimation of Oxygen
Oxygen, in an organic compound, can be calculated by the difference method.
Percentage of oxygen
Calculations of Empirical and Molecular Formula
Empirical Formula
It is the simplest whole number ratio between the atoms of various elements present in one molecule of the substance. eg. ratio of
Molecular Formula
It gives the actual number of atoms of various elements present in one molecule of the compound eg. actual no. of atoms of
Relationship between Empirical and Molecular formula
Molecular formula
Where
For example empirical formula of glucose is
Its empirical formula mass
Molecular Mass of glucose
Molecular formula of glucose
Steps to calculate Empirical formula
1. Devide the percentage of each element by its atomic mass to get the relative number of atoms in the molecule.
2. Devide the result obtained in the above step by the smallest value among them to get the simplest ratio of atoms of various elements present in the molecule.
3. If the ratio obtained in step 2 is not a whole no., then multiply the figures with a suitable integer to make it simplest whole number ratio.
4. Now write the symbols of the various elements present along with the whole numbers obtained in step 3. This gives the empirical or simplest formula.
Steps to determine Molecular formula
1. Determine empirical formula from percentage composition of atoms of various elements present.
2. Calculate empirical formula mass.
3. Calculate molecular mass by suitable method.
4. Determine value of
5. Multiply empirical formula by ’
Molecular formula
Empirical and Molecular formulae of some compounds
| Compound | Empirical Formula | Molecular Formula |
|---|---|---|
| Benzene | ||
| Glucose | ||
| Sucrose | ||
| Hydrogen peroxide | ||
| Carbon di oxide | ||
| Methane | ||
| Sodium Carbonate |
Solved problems
Question 1. Calculate empirical and molecular formula of an organic compound containing
Show Answer
Answer
| Element | Atomic Mass | Moles of atoms | Simplest atomic ratio | Simplest whole no. atomic ratio | |
|---|---|---|---|---|---|
| Carbon | 58.53 | 12 | 6 | ||
| Hydrogen | 4.06 | 1 | 5 | ||
| Nitrogen | 11.38 | 14 | 1 | ||
| Oxygen | 26.03 | 16 | 2 |
Therefore, the empirical formula is
Empirical formula mass
Empirical formula
Question 2. Calculate molecular formula of compound (Molecular mass
Show Answer
Answer (i) Calculation of empirical formula from
| Element | Atomic mass | Moles of atoms | Simplest atomic ratio | Simplest whole no. atomic ratio | |
|---|---|---|---|---|---|
| 54.2 | 12 | 2 | |||
| 9.2 | 1 | 4 | |||
| 36.6 | 16 | 1 |
Empirical formula
(ii) Calculation of molecular formula
Molecular formula
Question 3.
Show Answer
Answer (a) Calculation of
(i)
(ii)
(iii)
(iv) The given compound does not contain oxygen since the sum of
i.e.
(b) Calculation of empirical formula
| Element | Atomic mass |
Moles of atoms | Simplest atomic ratio |
Simplest whole no. atomic ratio |
|
|---|---|---|---|---|---|
| 21.95 | 12 | 2 | |||
| 4.58 | 1 | 5 | |||
| 73.37 | 80 | 1 |
Thus, the empirical formula of the compound is
(c) Determination of molecular formula
Vapour density of the compound
Molecular mass of the compound
Question 4.
Show Answer
Answer. Volume of acid taken
Volume of alkali used for neutralization of excess acid
Further
But,
Therefore,
Question 5.
Show Answer
Answer.
Question 6.
Show Answer
Answer. By formula,
Question 7.
Show Answer
Answer. By formula, \% of
Question 8.
Show Answer
Answer. By formula,
Atmospheric pressure
Room temperature
Vapour pressure of water at
By putting the volume of nitrogen collected at STP, into the formula, we get
Question 9.
Show Answer
Answer (i)
Question 10.
Show Answer
Answer.
Question 11. Suggesta suitable method to separate :-
(a) Mixture of
(b) Mixture of benzene (b.p.353K) and toluene (b.p.384K)
(c) Sugar containing impurity of common salt.
(d) Acetone (b.p. 329K) and methanol (b.p.338K)
(e) Mixture of benzoic acid and naphthalene
Show Answer
Answer (a) o-nitrophenol is steam volatile while
(b) For benzene and toluene, boiling point difference
(c) Sugar containing impurity of common salt can be purified by crystallization using hot ethanol which dissolves sugar but not the common salt.
(d) Acetone and methanol can be separated by fractional distillation as their boiling points are very close to each other (difference in b.p.
(e) Crystallization with hot water; benzoic acid will dissolve while naphthalene remains insoluble.
Question 12. Give method for the purification of
(a) Camphor
(b) Nitrobenzene
(c) Kerosene having water impurity
Show Answer
Answer. (a) Camphor sublimes upon heating, thus can easily be purified by sublimation
(b) Nitrobenzene, having very high b.p. (483K), insoluble in water and containg nonvolatile impurities, can be purified using steam distillation
(c) Kerosene can be purified from impurities of water by solvent extraction using a separating funnel since both are immiscible
Practice Problems
Question 1. In the Lassaigne’s test for ’
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)Question 2. A mixture of liquid
(a) Simple distillation
(b) Steam distillation
(c) Fractional distillation
(d) Distillation under reduced pressure
Show Answer
Answer: (c)Question 3. The best and latest technique for isolation, purification and separation of organic compounds is
(a) Crystallization
(b) Distillation
(c) Sublimation
(d) Chromatography
Show Answer
Answer: (d)Question 4. In which out of the following compounds, ’
(a) Nitrobenzene
(b) Aniline
(c) Urea
(d) Hydrazine
Show Answer
Answer: (d)Question 5. Which of the following compounds is / are formed when an organic compound containing both
(a) Thiocyanate
(b) Nitrate and sulphide
(c) Cyanide and sulphite
(d) Sulphide & cyanide
Show Answer
Answer: (d)Question 6. In Duma’s method, ’
(a)
(b) Gaseous nitrogen
(c) Ammonium sulphate
(d) Gaseous ammonia
Show Answer
Answer: (b)Question 7. The concentration of
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)Question 8. An organic compound decomposes below its boiling point, can be purified by
(a) Simple distillation
(b) Sublimation
(c) Distillation under reduced pressure
(d) Fractional distillation
Show Answer
Answer: (c)Question 9. Camphor can be purified by
(a) Crystallization
(b) Sublimation
(c) Distillation
(d) Extraction
Show Answer
Answer: (b)Question 10. The appearance of blood red colouration during Lassaigne’s test indicates
(a) Presence of
(b) Presence of
(c) Presence of
(d) Presence of halogen
Show Answer
Answer: (c)Question 11. Identify the compound that con not be Kjeldahlised
(a) Urea
(b) Pyridine
(c) Aniline
(d) Benzamide
Show Answer
Answer: (b)Question 12. Which of the following fertilizers has the highest percentage of ’
(a) Ammonium sulphate
(b) Urea
(c) Ammonium nitrate
(d) Calcium cyanamide
Show Answer
Answer: (c)Question 13. The Lassaigne’s extract is boiled with dil.
(a) Silver halides are soluble in
(b)
(c)
(d)
Show Answer
Answer: (d)Question 14. In the Kjeldahl’s method of estimation of ’
(a) 37.33
(b) 45.33
(c) 35.33
(d) 43.33
Show Answer
Answer: (a)Question 15.
(a) 19.25
(b) 18.15
(c) 19.01
(d) 20.2
Show Answer
Answer: (b)Question 16.
(a) 40.20
(b) 38.36
(c) 37.57
(d) 39.04
Show Answer
Answer: (c)Question 17.
(a) 38.75
(b) 50.23
(c) 40.57
(d) 37.85
Show Answer
Answer: (a)Question 18. A compound has
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)Question 19. 1.89g of an organic compound containg
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)Question 20. When acetic acid and lead acetate are added to sodium extract during Lassaigne’s test, appearance of black precipitate shows the presence of
(a) Phosphorus
(b) Nitrogen
(c) Sulphur
(d) Halogen
Show Answer
Answer: (c)Question 21. An organic substance from its aqueous solution can be separated by
(a) Simple Distillation
(b) Steam distillation
(c) Solvent extraction
(d) Fractional distillation
Show Answer
Answer: (c)Question 22. In steam distillation, the vapour pressure of the volatile compound is
(a) Equal to atmospheric pressure
(b) Less than atmospheric pressure
(c) More than atmospheric pressure
(d) Exactly half of the atomospheric pressure
Show Answer
Answer: (b)Question 23. Refining of petroleum involves the process of
(a) Simple distillation
(b) Steam distillation
(c) Distillation under reduced pressure
(d) Fractional distillation
Show Answer
Answer: (d)Question 24. In Kjeldahl’s method, the nitrogen present in the compound is converted into
(a) Gaseous ammonia
(b) Gaseous nitrogen
(c) Ammonium sulphate
(d) Ammonium nitrate
Show Answer
Answer: (a)Question 25. The property which serves as a criterion of purity of an organic compound its
(a) Solubility in water
(b) Conductivity
(c) Density
(d) Crystalline nature
Show Answer
Answer: (d)Question 26. Separation of two substances by fractional crystallization depends upon their difference in
(a) Densities
(b) Solubilities
(c) Melting points
(d) Boiling points
Show Answer
Answer: (b)Question 27. The separation of the constituents of a mixture by column chromatography depends upon their
(a) Different solubilities
(b) Different boiling points
(c) Different refractive index
(d) Differential adsorption
Show Answer
Answer: (d)Question 28. Ammonia evolved from
(a) 30.5
(b) 31.5
(c) 33.5
(d) 32.5
Show Answer
Answer: (b)Question 29. An organic compound contains carbon
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)Question 30. If two compounds have same Empirical formula but different Molecular formula, they must have
(a) Different percentage composition
(b) Same viscosity
(c) Different molecular weight
(d) Same vapour density
Show Answer
Answer: (c)Question 31. An oxide of nitrogen contains
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)Question 32. An acid contains
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)Question 33. A compound contains
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)Question 34. The most satisfactory method to separate sugars is
(a) Fractional crystallization
(b) Sublimation
(c) Chromatography
(d) Benedict’s reagent
Show Answer
Answer: (c)Question 35.
(a) 14.73
(b) 15.34
(c) 16.43
(d) 13.37










