Unit 07 Equilibrium Part-B
It is the study of equilibrium in the reactions where formation of ions takes place in aqueous solutions. The compounds which gives ions either in molten state or in solution are called electrolytes. The compound can be ionic (e.g. NaCl,
These electrolytes can be classified as
(i) Strong electrolytes
These electrolytes are almost completely ionised i.e. extent of dissociation is high
(ii) Weak electrolytes
These electrolytes dissociate in a solution to a small extent. The extent of dissociation is much less than one. These solution are poor conductors of electricity.
Note: This classification of electrolytes is based on their behaviour only in water as solvent.
The weak electrolytes can be categorized into three types
(a) Weak acids :
(b) Weak bases :
(c) Sparingly soluble salts:
Weak acids and weak bases
Consider ionisation of a weak electrolyte say a monoprotic acid, HA
where,
According to the law of equilibrium
where,
where,
Thus, degree of dissociation increases on dilution and this is known as Ostwald dilution law Similar expression can also be derived for a weak base
If
where,
The ion
At a given temperature
ACIDS AND BASES
Arrhenius Concept (1880 - 1890)
i. An Arrhenius acid is a substance which furnishes hydrogen ions (
ii. An Arrhenius base is a substance which furnishes hydroxyl
iii. The strength of an acid or base depends upon its tendency to furnish
iv. Water is amphoteric because it furnishes both
Limitations of Arrhenius concept
i. If fails to explain the behaviour of acids and bases in non-aqueous solvents.
ii. If fails to explain the neutralisation reactions giving rise to salt formation in absence of solvent.
e.g.,
Bronsted-Lowry Concept (1923)
i. A Bronsted acid is proton donor whereas, a Bronsted base is proton acceptor.
ii. The strength of the acids / bases depends upon the tendency to donate / accept the proton.
iii. Water is amphoteric because it donates as well as accepts proton.
iv. A pair of Bronsted acid-base which differ by a proton is known as conjugate pair of Bronsted acid and Bronsted base
Substance
Substance
Limitations
It does not explain the reactions between acidic oxides and basic oxides.
basic oxide acidic oxide
There is neither loss nor gain of a proton.
It could not explain the acidic nature of
Dissociation constant of conjugate acid-base pair
Let us consider, dissociation of weak acid HA in aqueous medium
Acid
Dissociation constant of conjugate base
From eqns. (i) and (ii)
where
Taking logarithm of Eqn. (iii)
Lewis concept (1939)
A base is defined as a substance which can furnish a pair of electrons to form a coordinate bond whereas an acid is a substance which can accept a pair of electrons.
i)
ii)
iii)
acid base
Lewis acids are
i. Molecules in which the central atom has incomplete octet:
ii. Simple cations:
iii. Molecules in which the central atom has empty d-orbitals :
The central atom of the halides such as
iv. Molecules having a multiple bond between atoms of dissimilar electronegativity:
Typical examples of molecules
Lewis Bases are
i. Neutral species having at least one lone pair of electrons :
For example, ammonia, amines, alcohols, etc. act as Lewis bases because they contain a pair of electrons.
ii. Negatively charged species or anions:
For example, chloride, cyanide, hydroxide ions, etc. act as Lewis bases.
It may be noted that all Bronsted bases are also Lewis bases but all Bronsted acids are not Lewis acids.
Limitations of Lewis concept
i. The important requirement of Lewis concept is the formation of a coordinate bond when a base reacts with an acid. However, well known acids such as
ii. The neutralisation process is usually fast but the formation of coordinate bond is slow.
Acid strength of simple cations increases with
i. Increase in +ve charge on the ion, i.e.,
ii. Adecrease in ionic radius
iii. An increase in effective nuclear charge for atoms
Note: The strength of cations as acids can alternatively be expressed in terms of effective nuclear charge. More is the effective nuclear charge more is the tendency to attract Ione pair of electron and thus, more is acidic nature, i.e.,
Acidic nature order :
iv. An increase in the number and relative electronegativity of the atom attached to central atom
v. Adecrase in atomic radius of the central atom.
However, these rules show some anomalies, e.g., acidic strength of boron trihalides is
Relative strength of acids and bases
The ratio of strengths of acids is known as relative strength, i.e.,
For weak acids
Relative strength for weak acids can be derived as follows :
Say acid
If concentrations are same, then
Self Ionisation of Water
Pure water is a weak electrolyte and dissociates as :
or
Before dissociation
After dissociation
Applying the law of equilibrium to dissociation equilibrium of water
where,
Since, dissociation of water is too less and thus,
or
The new constant
The value of
pH scale
This concept was given by Sorensen in 1909.
Just as
Considering the relationship
Taking log on both sides
or
pH scale
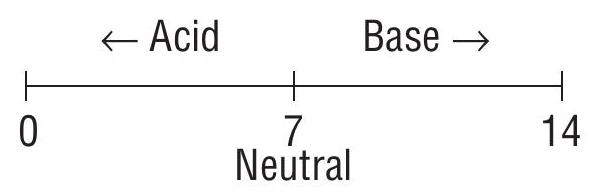
As temperature increases both
Calculation of
Let us consider ionization of weak monobasic acid HA; its degree of ionization is ’
Initial moles
Equilibrium moles
Dissociation constant of the acid may be calculated as ;
When
Calulation of
Let us consider, the ionization of weak base in aqueous medium
Dissociation constant of the base may be calculated as :
When
Question . 1 What is the
Show Answer
Answer .
c
Also,
This solution is to be diluted to make
Using
Originally, the solution was
Using
i.e. the given solution to be diluted to
Question . 2 Calculate
Show Answer
Answer . Both
millimoles of
Total moles of
Total volume of solution
Question . 3 Calculate the
Show Answer
Answer . As the solution is dilute (concentration
On solving the above quadratic equation
Now
However dilute the solution of an acid may be, it’s pH will always be less then 7 .
PRACTICE QUESTIONS
1. The conjugate acid of
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)2. The conjugate acid of
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)3. Conjugate base of Hydrazoic acid
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)4. Which of the following can act both as a Bronsted acid and a Bronsted base?
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)5. The
(a) 7
(b) 3
(c) nearly 7
(d) does not change
Show Answer
Answer: (b)6. An acid solution of
(a) 6.95
(b) 6
(c) 3
(d) 9
Show Answer
Answer: (a)7. Which of the following solution will have
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)8. The
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)9. A monoprotic acid (
(a) 11
(b)3
(c) 10
(d) 2
Show Answer
Answer: (a)10. The number of
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)11. The
(a) 1.89
(b) 2.89
(c) 4.89
(d) 2.19
Show Answer
Answer: (b)12.
(a) 2
(b) 7
(c) 5
(d) 12
Show Answer
Answer: (c)13. A
(a) 0.76
(b)1.26
(c) 1.76
(d) 2.26
Show Answer
Answer: (b)14. The
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)15. By adding
(a) 2
(b)1.3
(c) 0
(d)7
Show Answer
Answer: (b)16. The
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)HYDROLYSIS OF SALTS
Determination of pH due to Hydrolysis of Salts
The phenomenon of interaction of cations and / or anions of the salt with
There are four categories of salts
(i) Salt of a strong acid and a strong base.
Examples
(ii) Salt of a strong base and a weak acid.
Examples
(iii) Salt of a strong acid and a weak base.
Examples
(iv) Salt of a weak acid and a weak base.
Examples
Salts of strong acid and strong base do not undergo hydrolysis. They simply ionise and fail to change the
Salts of weak acid and strong base
Such salts give alkaline solution in water.
(i) Hydrolysis constant
Representing the salt by BA, the hydrolysis may be represented as follows :

or
or
i.e. it is a case of anion hydrolysis.
The hydrolysis constant
For the weak acid
The dissociation constant
Further the ionic product of water
Multiplying equation (i) with (ii) and dividing by (iii), we get
or
(ii) Degree of Hydrolysis
Suppose the original concentration of the salt in the solution is
Original molar conc.
Molar conc. at equilibrium
c
The hydrolysis constant
If
Substituting the value of
(iii)
(In the present case, we have)
Original conc.
conc. at equilibrium
C
i.e.,
Substituting the value of ’
or
or
Thus knowing the molar concentration ’
2. Salts of strong acid and weak base
(I). Hydrolysis constant
For the salt BA, the hydrolysis may be represented as :
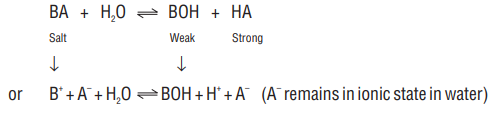
or
i.e. it is a case of cation hydrolysis.
The hydrolysis constant
For the weak base
The dissociation constant
The ionic product of water
Multiplying equation (vii) with (viii) and dividing by (ix), we get
(ii) Degree of Hydrolysis
Proceeding as in the earlier case, we get the same relationship viz.
Substituting the value of
(iii)
(In this case, we have)
Substituting the value of ’
or
3. Salts of weak acid and weak base
(i) Hydrolysis constant
Representing the salt by
or
i.e. it involves both anion hydrolysis as well as cation hydrolysis. Writing the various equation as before, we have
For the weak acid,
For the weak base,
Also, we know that
Multiplying equation (iii), (xiv), (xv) and dividing by equation (xvi), we get
(ii) Degree of hydrolysis.
In this case, we have
Original conc.
Molar conc. at equilibrium
Here, the relationship between ’
If ’
(iii)
for the weak acid
Butfrom equation (xviii),
or
The degree of hydrolysis and
Note:
Degree of hydrolysis always increases with increase in temperature as because at elevated temperature the increase in
Weaker is the acid or base, greater is the hydrolysis constant.
Degree of hydrolysis increases when ’
For any acid and its conjugate base or vice-versa
Question . 4 At
Show Answer
Answer . Volume of acid required to reach the equivalence point.
Concentration of salt
The salt so formed is a combination of weak base and strong acid
Also
or
Question . 5 Equal volume of two solution having
Show Answer
Answer . Given
Addition of equal volumes of acid and base results in the formation of neutral solution. The
PRACTICE QUESTIONS
1. The dissociation constant of
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)2.
(a) 3
(b)6.5
(c) 7
(d) 10
Show Answer
Answer: (c)3. Dissociation constant of
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)4. If
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)5. An aqueous solution of carbonic acid
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)6. The
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)7. Which of the following salt solutions (
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)8. A weak acid
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)9.
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)10. What is the percentage hydrolysis of
(a) 2.48
(b)5.26
(c) 8.2
(d)9.6
Show Answer
Answer: (a)11. The
(a) 2.4
(b)5.4
(c) 7.4
(d)9.4
Show Answer
Answer: (d)12. Ammonium cyanide is a salt of
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)13. It is found that
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)14. The degree of hydrolysis of ammonium acetate is related to its hydrolysis constant by the expression
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)15. The hydrolysis constant of anilinium acetate at
(a) 1.0
(b)1.21
(c) 1.35
(d)
Show Answer
Answer: (b)16. The expression to compute pH of a solution containing a salt formed from a weak acid and a weak base is
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)17. The
(a)
(b)6.5
(c) 4.3
(d) 3.94
Show Answer
Answer: (a)BUFFER SOULTIONS
A solution whose
General Characteristics of a Buffer Solution
(i) It has a definite
(ii) Its
(iii) Its pH does not change on dilution.
Buffer solutions can be obtained
(i) by mixing a weak acid with its salt with a strong base, e.g.,
(a)
(b) Boric acid + Borax
(c) Phthalic acid + Potassium phthalate
(ii) by mixing a weak base with its salt with strong acid, e.g.,
(a)
(b) Glycine + Glycine hydrochloride
(iii) by a solution of ampholyte. The ampholytes or amphoteric electrolytes are the substances which show properties of both an acid and a base. Proteins and amino acids are the examples of such electrolytes.
(iv) by a mixture of an acid salt and a normal salt of a polybasic acid, e.g.,
Types of Buffers
(i) Acidic buffer : A weak acid with its conjugate base :
Boric acid + Borax;
Phthalic acid + Potassium phthalate;
Explanation of Buffer Action
Acidic buffer : Consider the case of the solution of acetic acid containing sodium acetate. Acetic acid is feebly ionised while sodium acetate is almost completely ionised. The mixture thus contains
When a drop of strong acid, say
When a drop of
Thus,
pH of a Buffer mixture solution (Henderson equation)
Consider a buffer mixture say an acidic buffer e.g. HA + NaA
Applying law of equilibrium to dissociation of
Where,
(ii) Basic buffer
A weak base with its conjugate acid :
Glycine + Glycine hydrochloride .
Blood buffer contain
Explanation of Buffer Action
Basic buffer : Consider the case of the solution containing
When a drop of
(From strong base)
When a drop of
Thus, pH of the buffer is practically unaffected.
pH of a Buffer-Mixture solution (Henderson Equation)
It consists of a mixture of weak base and its salt (strong electrolyte). The ionisation of weak base
Applying law of equilibrium to dissociation of
Where,
Utility of Buffer solutions in analytical chemistry
Buffers are used:
(i) To determine the
(ii) For the removal of phosphate ion in the qualitative inorganic analysis after second group using
(iii) For the precipitation of lead chromate quantitatively in gravimetric analysis, the buffer,
(iv) For precipitation of hydroxides of third group of qualitative analysis, a buffer,
(v) A buffer solution of
Buffers are used in industrial processes such as manufacture of paper, dyes, inks. paints, drugs, etc. Buffers are also employed in agriculture, dairy products and preservation of various types of foods and fruits.
Buffer capacity and Condition for maximum buffer capacity
Buffer capacity
The property of a buffer solution to resist alteration in its
Buffer capacity
Thus greater the buffer capacity, the greater is the capacity of the buffer to resist change in
Question . 7 How many grams of sodium acetate are required to prepare buffer solution of
Show Answer
Answer . A mixture of acetic acid and sodium acetate is an acidic buffer
Using Henderson equation
It is difficult to take anti log of negative number, rather than above equation we may use the same relation in the form.
or
mass of salt
The addition of water (
Question . 8
Show Answer
Answer . Since the two solution are mixed in equal volumes therefore, the concentration of
The presence of strong acid in a mixture suppresses the ionization of weak acid therefore, before the start of the titration,
After neutralization of
For a weak acid
Change in
The
PRACTICE QUESTIONS
1. One litre of a buffer solution containing
(a) 9
(b) 10
(c) 4
(d) 6
Show Answer
Answer: (b)2. The approximate
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)3. A certain buffer solution contains equal concentration of
(a) 3
(b) 8
(c) 11
(d) 14
Show Answer
Answer: (b)4. The
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)5. A certain buffer solution contains equal concentrations of
(a) 4
(b) 10
(c) 7
(d) 14
Show Answer
Answer: (a)6. In a mixture of a weak acid and its salt, the ratio of the concentration of acid to salt is increased tenfold. The pH of the solution
(a) decreases by one
(b) decreases by one tenth
(c) increases by one
(d) increases ten-fold
Show Answer
Answer: (a)7. How much sodium acetate should be added to
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)8. For preparing a buffer solution of
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)9. A buffer solution is prepared by mixing
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)10. What is
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)11. The
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)12. Solution of
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)13. The most important buffer in the blood consists of:
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)14. The pH of blood is maintained by the balance between
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)SOLUBILITY PRODUCT
Solution which remains in equilibrium with undissolved solute (salt) is said to be saturated. At saturation stage, the quantity of the salt dissolved is always constant, for the given amount of a particular solvent at a definite temperature. This is referred to as the solubility of the given salt at the given temperature.
In a saturated solution of a salt the following equilibrium exists :
Applying the law of equilibrium to the ionic equilibrium,
Since, the concentration of the solid salt is constant at a particular temperature, i.e.,
Hence,
Relationship between Solubility and Solubility Product
The equilibrium for a saturated solution of any sparingly soluble salt may be expressed as :
Thus, solubility product,
Let ’
So,
(i) 1:1 (AB) type salts:
Examples:
Binary electrolyte :
Let solubility of
So,
or
(ii)
Examples:
Ternary electrolyte:
Let solubility of
So,
or
Let ’
or
(iii) 1:3 (AB) type salts:
Examples:
Quaternary electrolyte:
Let ’
The presence of a common ion affects the solubility of a salt. Let
Before addition of
After addition of
So,
Equating Eqns. (i) and (ii)
Thus, the solubility of the salt decreases due to common ion effect
Important Cases of Calculating Solubility of a Salt
(i) Solubility of a salt of strong acid and strong base
(a) in the absence of common ion
(b) In the presence of common ion say
(ii) Solubility of a salt of weak acid and strong base
(a) In pure water
since it is a salt of weak acid and strong base, it will undergo anionic hydrolysis
Since the concentration of
(b) In the presence of an acid
The following equilibrium is also quickly established
Since
(c) in the presence of basic buffer
Here, hydrolysis of
Thus, solubility of such salts is maximum in acidic solution, then in pure water and then in basic solution.
(iii) Solubility of a salt of strong acid and weak base
Such salts have maximum solubility in a basic solution then in pure water and then in an acidic solution.
(iv) Solubility of a salt of weak acid and weak base
in pure water
on hydrolysis
Solubility of such salts would increase in presence of both, an acid or a base.
Criteria of precipitation of an electrolyte
A very useful conclusion is derived from the solubility product concept. No precipitation of the electrolyte occurs if the ionic product
Case I: When
Case II: When
Case III: When
When the ionic product exceeds the solubility product, the equilibrium shifts towards left hand side resulting in precipitation of the salt.
Thus, for the precipitation of an electrolyte, it is necessary that the ionic product must exceed its solubility product. For example, if equal volumes of
In the resulting solution,
and
lonic product of
Applications of solubility product
(i) Salting out of soap:
Soap is a sodium salt of higher fatty acids. From the solution, soap is precipitated by the addition of concentrated solution of sodium chloride. Soap and sodium chloride are present in the form of ions.
Thus, the concentration of
(ii) Precipitation of the sulphides of group II and IV:
Hydrogen sulphide is a weak electrolyte and is used for the precipitation of various sulphides of group II and IV in qualitative analysis.
Passing
Now
From (i),
So if we pass
Passing
Now if we pass
Note that in a saturated solution of
In this way, we now know the concentration of sulphide ions in an acidic solution, so accordingly we can plan precipitation of metal ions. The important aspect of this method is that we can always increase or decrease the concentration of sulphide ion by controlling the amount of acid. In acidic medium (II group),
(iii) Precipitation of hydroxides of Group III:
Ammonium hydroxide is a precipitating agent in Group III of the qualitative analysis. It is a weak electrolyte and ionises in water as
The concentration of
In order to precipitate only the hydroxides of group III, the
(v) Using excess of precipitating agent in gravimetric analysis:
So as to ensure complete precipitation in gravimetric analysis, an excess of precipitating agent is always used. In gravimetric analysis, barium is precipitated as barium sulphate. No doubt,
Question. 9 The solubility of
Show Answer
Answer . Loss in weight of
The presence of
Given solubility
Let ’
lonic product
At equilibrium
Question. 10 The solubility product
Show Answer
Answer . The first step is to determine the concentration of
Ionic product of salt in the solution
At this stage
i.e.
Question. 11 What is the solubility of
Given
Show Answer
Answer .
Let the solubility of the salt be
The
Here,
Also,
Assuming
On solving (i) and (ii) we get
PRACTICE QUESTIONS
1. If equal volumes of
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)2. The solubility of solid silver chromate,
I. pure water
II.
III.
Predict the relative solubility of
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)3. The solubility products of
(a)
(b)
(c) Both together
(d) None of these
Show Answer
Answer: (a)4. If
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)5.
(a) The solvent becomes more polar on adding
(b)
(c)
(d)
Show Answer
Answer: (d)6. Why does only
(a) The solubility product of
(b) Enough
(c) Azinc salt does notionize in an acidic medium
(d) The solubility product changes
Show Answer
Answer: (a)7. When
(a)
(b) The ionic product
(c) The
(d) The ionic product
Show Answer
Answer: (b)8. Excess
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)9. A solution is found to contain
Given:
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)10. A saturated solution of
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)11. The solubility of
(a)
(b)
(c)
(d)
Read the passage and answer the questions given below :
In qualitative analysis, cations of group II as well as group IV both are precipitated in the form of sulphides. Due to low value of
In the solution containing
Show Answer
Answer: (c)12. If
(a)
(b) CdS
(c)
(d) SnS & CdS (both together)
Show Answer
Answer: (a)13. At what value of
(a)
(b) 7
(c)
(d) 4
Show Answer
Answer: (c)14. Which of the following sulphide is more soluble in pure water?
(a)
(b) NiS
(c)
(d) Equal solubility for all
Show Answer
Answer: (b)15. If
(a)
(b)
(c)
(d)










