Unit 06 Chemical Thermodynamics
Thermodynamics: Means flow of heat
One of the fundamental subject in physical chemistry
It’sa science of macroscopic properties i.e. properties of matter in bulk
It deals with energy changes accompanying all types of physical and chemical processes
It predicts the feasibility or spontaneity of a process, including a chemical reaction, under a given set of conditions
It helps to determine the extent to which a process can proceed before attainment of equilibrium
Terms used in Thermodynamics
System : Part of universe chosen for thermodynamic studies.
Surroundings: Remaining universe other than the system.
Universe
Boundary: Real or imaginary surface that separates the system from its surroundings
Classification of Systems
On the basis of possibility of movement of matter and energy in or out of the system.
(a) Open System: It can exchange both matter and energy with its surroundings.
e.g. tea kept in on open vessel.
(b) Closed System : It can exchange only energy with its surroundings but not matter.
e.g. tea kept in closed vessel.
(c) Isolated System : It can neither exchange matter nor energy with its surroundings.
e.g. tea kept in thermos flask.
State of the System
A system is said to be in a definite state if each of its measurable property has a definite value.
A system is described by specifying some or all of its
State of the system is specified by state functions or state variables.
Types of Function / Variables
(a) State Function
A physical quantity whose value depends upon the state of the system and does not depend upon the path by which the state has been attained.
e.g. p,
(b) Path Function
A physical quantity which depends on the path by which change is brought.
e.g. work and heat.
Macroscopic Properties of the System
(a) Intensive Properties
Properties which do not depend upon the amount of the substance. e.g. pressure, density, temperature, surface tension, boiling point, refractive index etc.
(b) Extensive Properties
Properties which depend upon the amount of the substance.
e.g. volume, mass, heat capacity, number of moles etc.
(c) Molar Property
Value of an extensive property ’
All the molar properties are intensive properties.
Types of Processes
(a) Reversible Process
A process which is carried out infinitesimally slowly by a series of steps such that system and surroundings are always in near equilibrium with each other.
At any moment, the process can be reversed by an infinitesimal change.
(b) Irreversible Process
A process which cannot be reversed by small change.
Irreversible processes are carried out at finite rates.
(c) Adiabatic Process
A process during which no heat can flow in or out of the system.
(d) Isothermal Process
It is carried out at constant temperature
(e) Isobaric Process
It is carried out at constant pressure
(f) Isochoric Process
During the process, volume of the system remains constant
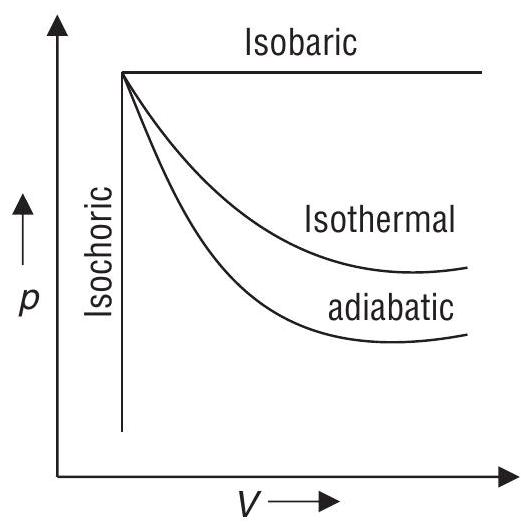
(g) Cyclic Process
When a system undergoes a series of changes and finally returns to its initial state.
Common modes of transfer of energy between system and surroundings
Internal Energy (U)
It is the sum of all types of energies, a system may have like chemical, electrical, mechanical or any other type of energy.
It is a state function and an extensive property
Absolute measurement of
where,
U of the system may change, when
Heat passes into or out of the system
Work is done on or by the system
Matter enters or leaves the system
At constant volume
Sign Convention
If energy is released,
If energy is absorbed,
Work(w)
A form of energy transferred from system to the surroundings or from surroundings to the system due to difference in some property (other than temperature) between system and surroundings
Types of work
Mechanical work (due to difference in pressure)
work of expansion or compression or
Mechanical work
Electrical work (due to difference in electrical potential)
Sign Convention
If work is done on the system,
If work is done by the system,
Heat
It is a form of energy exchanged between system and surroundings due to difference in temperature between system and surroundings
Sign Convention
If heat is absorbed (endothermic reaction),
If heat is released (exothermic reaction),
Units of work, heat and energy
SI unit of work
Cgs unit of work
Work done (w) in various processes
Work of expansion / compression or
a) Work done in irreversible isothermal expansion against a constant external pressure
b) Wore done in isothermal reversible expansion of
(-ve sign has been used as it is work of expansion i.e. work done by the system)
c) Free Expansion (Expansion in vaccum)
Ideal gas:
Real gas:
Work and heat are not state functions, they are path functions
First Law of Thermodynamics
The energy of an isolated system is constant. Mathematically
This law is also known as law of conservation of energy i.e. energy can neither be created nor destroyed
Isothermal and Free Expansion of an Ideal Gas
Also,
1. For isothermal irreversible change
2. For isothermal reversible change
3. For adiabatic change,
Comparison of reversible, irreversible and adiabatic work
For reversible expansion,
Enthalpy
It is the heat evolved or absorbed at constant pressure.
It is a state function and an extensive property.
For exothermic reactions,
For endothermic reactions,
Also,
where,
The difference in
Heat capacity (C)
Amount of heat required to raise the temperature of the system by
It is an extensive property
Units :
Specific Heat Capacity (c)
Amount of heat required to raise the temperature of one gram (unit mass) of a substance by
It is an intensive property
Units :
where,
Specific heat capacity of water is
Molar heat capacity (
Amount of heat required to raise the temperature of 1 mole of the substance by
where,
Unit: J mol
It is an intensive property
Types of Heat Capacities
For an ideal gas
For
Importance of
Ratio of
Measurement of
Since,
So,
In this case, the heat lost or gained
If reaction involves only solids & liquids or
For a cyclic process :
During isothermal expansion of an ideal gas,
Enthalpy change of the reaction
The amount of heat absorbed or evolved in a chemical reaction when moles of reactants and products being the same as indicated by the balanced chemical reaction.
Standard Conditions
Pressure
Temperature
The standard state of a substance at a specified temperature is its pure form at 1 bar.
Standard conditions are denoted by adding the superscript 0 or
Standard enthalpy of a reaction
The enthalpy change accompanying the reaction when all the reactants and products are taken in their standard states ( 1 bar pressure and at any specified temperature, generally
Depending upon the nature of the reaction, enthalpy of the reaction is named accordingly.
Enthalpy of combustion,
The enthalpy change when one mole of a substance is burnt completely in excess of oxygen.
Enthalpy of formation
The enthalpy change when one mole of a substance is formed from its elements under given conditions of temperature and pressure.
Enthalpy of neutralization
The enthalpy change when one gram equivalent of the acid is neutralized by a strong base.
Enthalpy of solution
The enthalpy change when one mole of the substance is dissolved in a specified amount of the solvent.
For most of the ionic compounds
Enthalpy of Hydration
Amount of heat evolved or absorbed when one mole of an anhydrous salt combines with the required number of water molecules to form the hydrated salt.
Dissociation process of the compounds is endothermic
Solubility of most salts in
Enthalpy of atomization
The enthalpy change when one mole of a substance dissociates into gaseous atoms.
Enthalpy of Reaction
For example, the most stable states of oxygen, bromine, iodine are
whereas for carbon
Depending upon the type of process involving a phase change, the enthalpy change (for one mole of a substance) is named accordingly.
Enthalpy of fusion,
Enthalpy change that accompanies melting of one mole of a solid substance at constant temperature and pressure.
Melting of a solid is endothermic process
Enthalpy of vaporization
Amount of heat required to vaporize one mole of a liquid at constant temperature and pressure.
Enthalpy of sublimation
Enthalpy change when one mole of a solid substance sublimes at a constant temperature and pressure.
Note: Magnitude of enthalpy change depends on the strength of the intermolecular interactions in the substance undergoing the phase transformations.
Hess’s Law of constant heat summation
the total enthalpy change accompanying a chemical reaction is the same whether the reaction takes place in one or more steps.
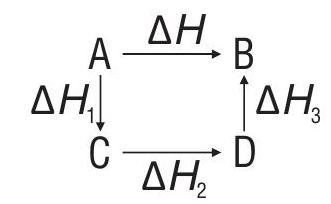
When chemical equations are added, subtracted or multiplied, the enthalpy changes can also be added, subtracted or multiplied.
The sign of
Bond enthalpy
The amount of energy required to dissociate one mole of bonds present between the atoms in the gaseous molecules. Two separate terms are used in thermodynamics (i) bond dissociation enthalpy (ii) mean bond enthalpy.
Bond dissociation enthalpy is the change in enthalpy when one mole of covalent bonds of a gaseous compound is broken to form products in gas phase.
Mean bond enthalpy is the average value of bond dissociation enthalpy of a particular type of bond present in different compounds.
Units:
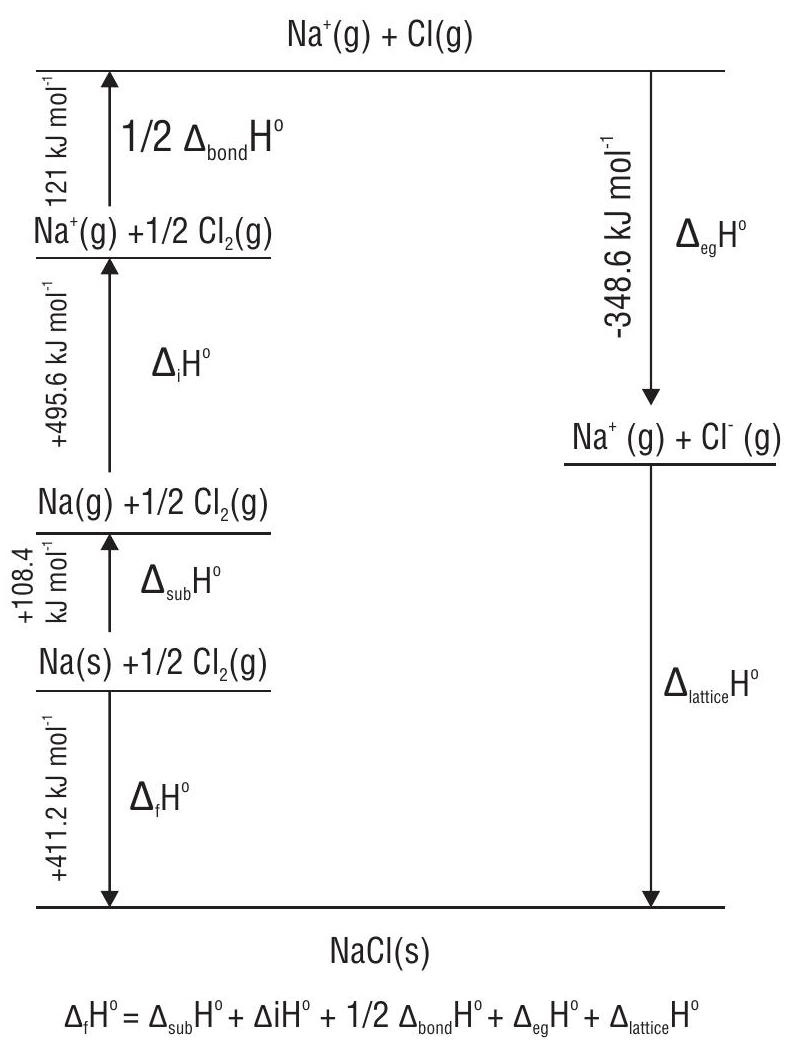
Calculation of lattice enthalpy using Born-Haber cycle
Lattice enthalpy
The sum of the enthalpy changes round a Born-Haber cycle is zero.
Enthalpy diagram for lattice enthalpy of NaCl (Born Haber cycle for NaCl).
Spontaneous process : a physical or chemical change that occurs on its own. It may or may not need initiation.
Non-spontaneous process : a process which cannot take place by itself nor by initiation.
Two factors which govern the spontaneity of a process:
-
Tendency to attain minimum energy state.
-
Tendency to attain maximum randomness or disorder.
First law of thermodynamics puts no restriction on the direction of heat flow or direction of any process.
The flow of heat is unidirectional from higher temperature to lower temperature.
Most of the processes occur spontaneously only in one direction under a given set of conditions of temperature and pressure.
Decrease in enthalpy is not the only criterion for spontaneity.
Entropy,
It is a state function i.e. path independent
Qualitatively,
A crystalline solid will have the lowest entropy, whereas the gaseous state will have the highest entropy.
The order of randomness and thus, the entropy is:
Gas > liquid > solid
For a reversible change at constant temperature
Unit:
In achemical reaction:
Entropy changes during phase transformations
(a)
(b)
(c)
For all spontaneous processes, the total entropy change must be positive
When a system is in equilibrium, the entropy is maximum and the change in entropy,
Second Law of Thermodynamics
For a spontaneous process in an isolated system, the change in entropy is positive. Or
It is impossible to convert heat completely into work without leaving some effects elsewhere.
Gibbs Energy,
The maximum amount of energy available to a system, during a process, that can be converted into useful work.
It is a state function and an extensive property
Units:
Gibbs Helmholtz Equation
If,
If,
It,
Relationship between equilibrium constant
Relationship between electrical work done in the galvanic cell and
If reactants and products are in their standard states
Effects of signs of
| Sign of |
Sign of |
Value of |
Spontaneity |
|---|---|---|---|
| - | + | Any | Spontaneous |
| + | - | Any | Non-spontaneous |
| - | - | Low | Spontaneous |
| - | - | High | Non-spontaneous |
| + | + | Low | Non-spontaneous |
| + | + | High | spontaneous |
Solved Examples
Question 1. How many calories are required to heat
(a) 120
(b) 2400
(c) 1200
(d) 180
Show Answer
Solution: d
For a monoatomic gas,
40 gram of Argon
Heat required to raise the temperature from
Question 2.
(a)
(b)
(c)
(d)
Show Answer
Solution:
Question 3. The standard enthalpy of formation of
(a)
(b)
(c)
(d)
Show Answer
Solution:
Enthalpy of formation of
(b)E.
- There are
Question 4. The standard molar enthalpies of formation of cyclohexane (I) and benzene (I) at
(a)
(b)
(c)
(d)
Show Answer
Solution:
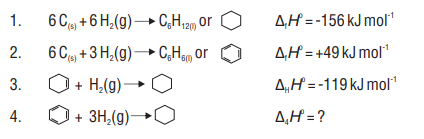
Enthalpy change for the hydrogenation of benzene should be three times the enthalpy of hydrogenation of cyclohexene
On adding equations (2) and (4), we get
(5)
This reaction also gives the energy of formation of cyclohexane.
The difference in the enthalpy of formation calculated by these alternate paths is due to resonance stabilization of benzene.
Resonance energy of benzene
Question 5. The enthalpy of formation of
(a)
(b)
(c)
(d)
Show Answer
Solution:
1.
2.
3.
4.
5.
First, calculate the enthalpy of combustion of propene using Hess’s law.
i.e.
6.
Practice Questions
Question 1. For which of the process,
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)Question 2. The favourable conditions for a spontaneous reaction are
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)Question 3. Certain reaction is at equilibrium at
(a) 55.0
(b) 60.0
(c) 68.5
(d) 120.0
Show Answer
Answer: (b)Question 4. Which of the following thermodynamic relation is correct?
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)Question 5. For a gaseous reaction
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)Question 6. The values of
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)Question 7. A reaction is spontaneous at low temperature but non-spontaneous at high temperature. Which of the following is true for the reaction?
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)Question 8.
(a)
(b)
(c)
(d) none of these
Show Answer
Answer: (c)Question 9. The enthalpy change for the reaction,
(a)
(b)
(c)
(d) None of these is correct
Show Answer
Answer: (a)Question 10. Two moles of an ideal gas is expanded isothermally and reversibly from 1 litre to 10 litre at
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)Question 11. When 0.1 mole of a gas absorbs
(a) triatomic
(b) diatomic
(c) polyatomic
(d) monoatomic
Show Answer
Answer: (b)Question 12. An insulated container is divided into two compartments. One compartment contains an ideal gas at a pressure
(a)
(b)
(c)
(d) All the three are true
Show Answer
Answer: (d)Question 13. One mole of a non-ideal gas undergoes a change of state
(a) 44.0
(b)
(c) 44.0
(d) not defined, because pressure is not constant
Show Answer
Answer: (c)Question 14. An ideal gas is allowed to expand both reversibly and irreversibly in an isolated system. If
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)Question 15. One mole of an ideal gas expands freely and isothermally at
(a)
(b)
(c) zero
(d)
Show Answer
Answer: (c)Question 16. Which one of the following equations does not correctly represent the first law of thermodynamics for the given process?
(a) Isothermal process:
(b) Cyclic process:
(c) Isochoric process :
(d) Adiabatic process :
Show Answer
Answer: (d)Question 17. When
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)Question 18. Work done in expansion of an ideal gas from
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)Question 19. The bond dissociation energies of
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)Question 20. Under which of the following condition is the relation
(a) constant pressure
(b) constant temperature
(c) constant temperature and pressure
(d) constant temperature, pressure and composition
Show Answer
Answer: (a)Question 21. Given
The heat of formation of
(a)
(b)
(c)
(d) None
Show Answer
Answer: (a)Question 22. Given that the enthalpy of formation of
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)Question 23.
From the above data, the standard heat of formation of
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)Question 24. Given:
Bond energies :
The energy of
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)Question 25. The bond energies of
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)Question 26. Enthalpy of
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)Question 27. When 1 mole of crystalline
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)Question 28. The lattice enthalpy and hydration enthalpy of four compounds are given below:
| Compounds | Lattice enthalpy |
Hydration enthalpy (in |
|---|---|---|
| P | +780 | -920 |
| Q | +1012 | -812 |
| R | +828 | -878 |
| S | +632 | -600 |
The pair of compounds which is soluble in water is
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)Question 29. Given the following data:
Heat of atomisation of
Heat of atomisation of
Bond energy of
What is
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)Question 30. If an endothermic reaction occurs spontaneously at constant
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)Question 31. For the given reaction
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)Question 32. Consider the reaction
If
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)Question 33. Oxidizing power of chlorine in aqueous solution can be determined by the parameters indicated below:
The energy involved in the conversion of
(Using the data,
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)Question 34. Standard entropy of
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)Question 35. For a particular reversible reaction at temperature
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)Question 36. The value of enthalpy change
at
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)Question 37. The incorrectexpression among the following is:
(a)
(b)
(c) In isothermal process,
(d)
Show Answer
Answer: (d)Question 38. A gas present in a cylinder fitted with a frictionless piston expands isothermally against a constant pressure of 1 bar from a volume of
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)Question 39. A system containing an ideal gas was subjected to a number of changes as shown in the
Temperature at
Temperature at
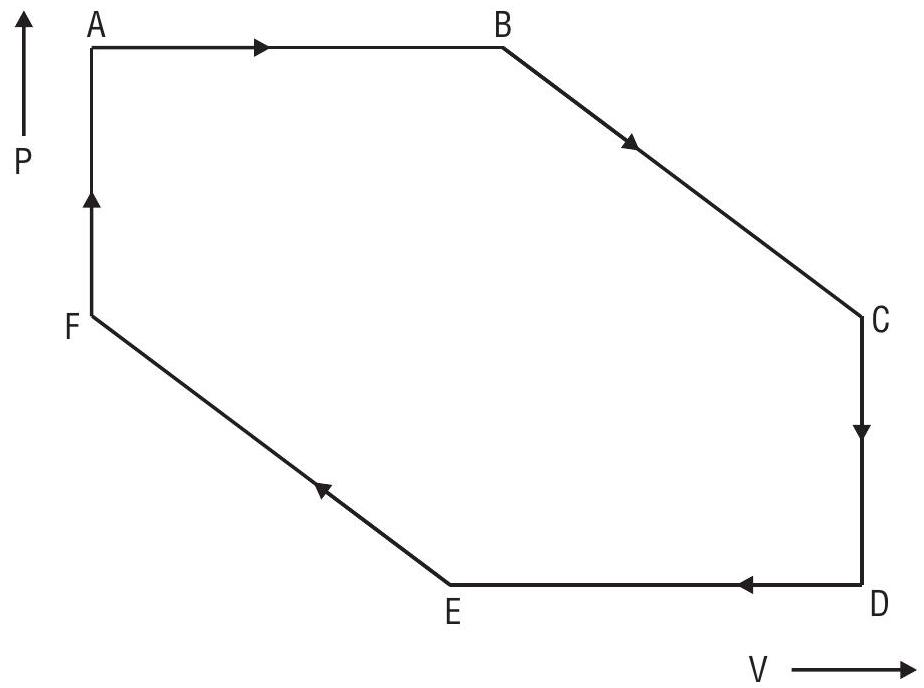
(a) Isochoric and isothermal (pressure falls)
(b) Adiabatic compression, temperature increases to
(c) Adiabatic expansion, temperature fall to
(d) Isochoric and isothermal (pressure increases)
Show Answer
Answer: (c)Question 40. In the reactions
I:
II:
III :
(a) 2.8
(b) 1.2
(c) 0.36
(d) 0.82










