Unit 05 State Of Matter: Gas And Liquids
General Idea
Matter can exist (mainly) in three states viz; solid, liquid and gas.
State of matter is determined by the nature of intermolecular forces, molecular interactions and thermal energy of particles.
Change in the physical state does not change the chemical properties of a substance.
Rates of Chemical reactions depend upon the physical state.
Physical laws govern the behavior of matter in different states.
Intermolecular Forces
Forces of attraction and repulsion between interacting particles (atoms and molecules).
Covalent bonding is not intermolecular force.
Van der Waals Forces
Attractive intermolecular forces.
These forces include dispersion forces or London forces, dipole-dipole forces and dipoleinduced dipole forces.
Ion-dipole forces are not van der Waals forces.
Dispersion forces or London Forces
Force of attraction between two temporary instantaneous dipoles.
There forces are always attractive.
Interaction energy
Dipole-Dipole Forces
Present between the molecules possessing permanent dipoles.
For stationary polar molecules : interaction energy
For rotating polar molecules : interaction energy
Hydrogen-bonding
Aspecial case of dipole-dipole interaction.
Exists in the molecules which are highly polar containing
Energy of
One of the important forces in proteins and nucleic acids.
It determines the structure and properties of many compounds.
Dipole-Induced Dipole Forces
Exists between polar molecules having permanent dipole and non-polar molecules.
Interaction Energy
Existence of the three states of matter - It is due to the balance between intermolecular forces and the thermal energy of the molecules.
Predominance of intermolecular interactions
Gas
Predominance of thermal energy
Gas
Comparison between gaseous and liquid states
| Gaseous State | Liquid State |
|---|---|
The Gas Laws
1. Boyle’s Law (
At constant
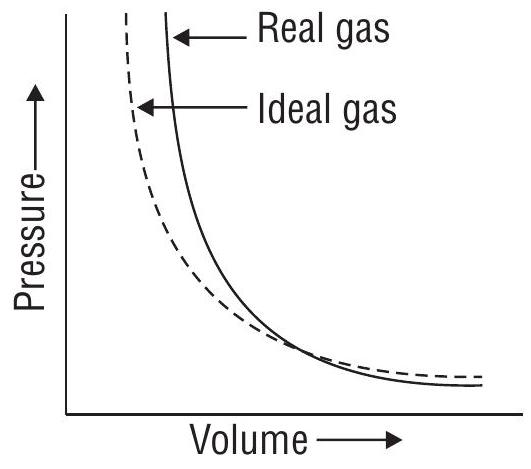
At a constant temperature, the pressure exerted by a fixed mass of a gas is inversely proportional to its volume.
Graphical Representation
Isotherm- line / plot between
Isobar- line / plot between
Isochore - line / plot between
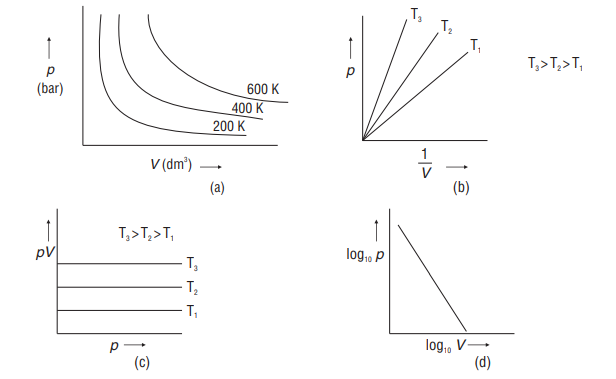
All the graphs shown above are isotherms
Note: At high pressures, gases deviate from Boyle’s law. So, a straight line is not obtained in the graph of
Relation between density
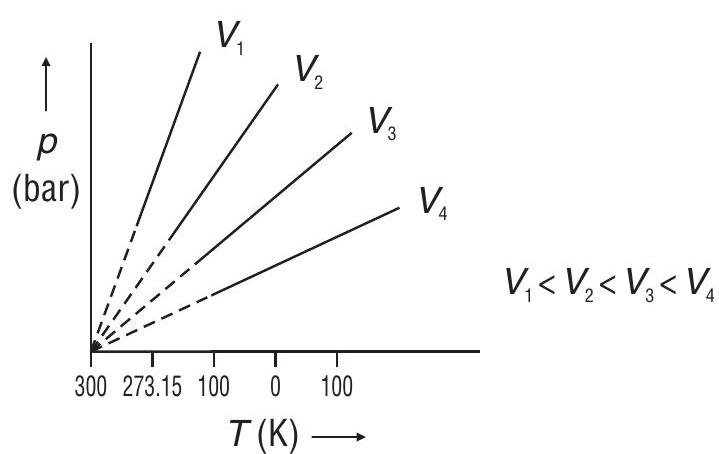
2.Charles’ Law (
At constant
i.e.
For a fixed mass of a gas, at a constant pressure, the volume of a gas is directly proportional to its absolute temperature.
Kelvin Temperature Scale or Absolute Temperature Scale
Also, known as thermodynamic scale of temperature.
Graphical Representation

The graphs shown above are isobars
Absolute Zero (the lowest possible temperature) - The lowest hypothetical or imaginary temperature at which gases are supposed to occupy zero volume. Absolute Zero is equal to
Note:
1. All gases obey Charles’ law at very low pressures and high temperatures.
2. Boyle’s and Charles’ law are examples of a limiting law, a law i.e. strictly true only in certain limit in this case
3.Gay Lussac’s Law (
Graphical representation
The graphs shown above are isochores.
4. Avogadro’s Law (
This law states that equal volumes of all gases under similar conditions of temperature and pressure contain equal number of molecules.
No. of molecules in one mole of a gas
Molar Volume-Volume occupied by one mole of each substance.
It contains the same number of molecules i.e.
Molar Volume at different
| Conditions | Temperature | Pressure | Molar Volume |
|---|---|---|---|
| NTP (Earlier also called STP) | |||
| STP | |||
| SATP | 1 bar |
STP : Standard Temperature and Pressure
NTP: Normal Temperature and Pressure
SATP: Standard Ambient Temperature and Pressure
No. of moles,
or
where,
Ideal gas equation or Equation of State
It describes the state of any gas
Combine Boyle’s law, Charles’ law and Avogadro’s law
5. Combined Gas Law:
Ideal gas equation in terms of density (Relationship between molar mass and density of a gas)
Values of the gas constant:
In the C.G.S. units
In S.I. units
In terms of calories
1.987 calories
Dalton’s Law of Partial Pressures :
Law is valid for non-reacting gases, at constant volume & constant Temperature.
where,
Gases are generally collected over water and therefore are moist.
Aqueous Tension : Pressure exerted by saturated water vapour.
Partial Pressure in terms of mole fraction
where,
Different Pressure units and their relation
1 bar
1 Torr
Psi = Pound per square inch
Standard pressure
Normal pressure
Graham’s Law of diffusion / effusion
At constant
where
As molar mass is directly proportional to the density of the gas at constant
where
If two gases are taken at different pressures, then, as greater the pressure, greater is the number of molecules hitting per unitarea, greater is the rate of diffusion.
For two gases undergoing diffusion at the same pressure but at two different temperatures:
Comparison of volumes of two different gases effused / diffused during the same time interval
where
the same time
Comparison of times taken for effusion / diffusion of the same volume of two different gases
where,
for effusion / diffusion of the same volume under the same conditions of temperature and pressure.
Kinetic Molecular Theory of Gases :
This theory helps us to understand the behavior of gases. This theory provides a microscopic model of gases.
Postulates / Assumptions
Gases consist of large number of very small identical particles (atoms or molecules) which are so for apart on the average that the actual volume of the molecules is negligible as compared to the empty space between them.
This explains the high compressibility of gases
No force of attraction between the particles of a gas.
Particles, within the container, are in ceaseless random motion during which they collide with each other and with the walls of the container.
All the collisions are perfectly elastic i.e. total energy of molecules before and after the collision remains same. The energy may, however, be transferred from one molecule to the other on collision.
Due to the bombardment of the molecules on the walls of the containing vessel, pressure is exerted by the gas on the walls of the containing vessel.
Particles of the gas move in straight line (i.e. it obeys Newton’s first law of motion).
At any particular time, different particles in the gas have different speeds and hence different kinetic energies.
Average K.E. of the gas molecules is directly proportional to the absolute temperature.
i.e. Molecular velocity of any gas is directly protional to the square root of temperature.
Kinetic Gas Equation
Different Type of Speeds possessed by molecules
Most probable speed
speed possessed by maximum number of molecules.
Average speed
Average speed possessed molecules of a gas at a given temperature.
Root mean square speed
Square root of mean of the squares of the speeds of different molecules of the gas at a given
Comparison of different speeds
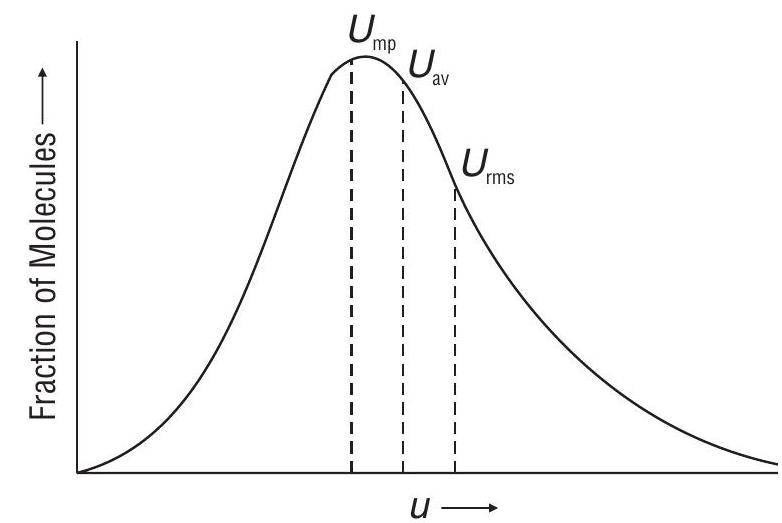
Maxwell - Boltzmann distribution of molecular speeds
Another relation between speeds
Ideal and Real Gas
Ideal gas
A gas which obeys ideal gas equation,
Real gas
A gas which obey gas laws at low pressure and high temperature. All gases are real gases.
Causes of Deviation from Ideal Behaviour
Assumptions of Kinetic theory of gases which do not hold good in all conditions:
1. The intermolecular force of attraction between gaseous molecules is negligible.
2. The volume occupied by the gas molecules is negligible in comparison to the total volume of the gas.
Compressibility Factor
Plot : variation of
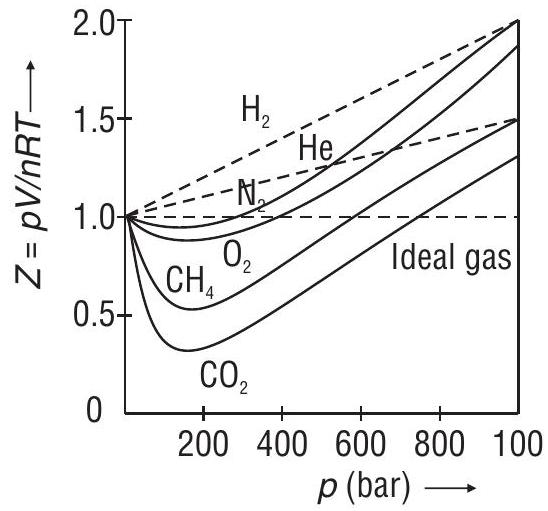
The value of
Plot of
The variation of
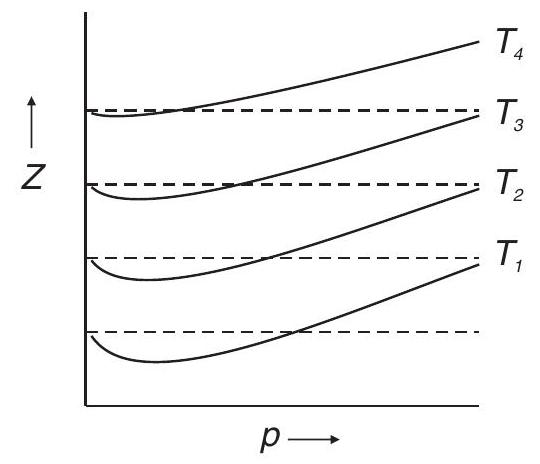
The deviation from ideal behavior becomes less and less with increase in temperature.
Boyle’s temperature :
Temperature at which a real gas obeys ideal gas equation over an appreciable range of pressure.
Significance of compressibility factor
for the ideal gas,
van der Waals equation
This equation explains the behavior of real gases (not completely).
It considered two facts 1. Attraction between molecules
Corrected Corrected
Pressure Volume
I Term :

II Term: Corrected volume
where,
Also,
where,
Significance of van der Waals constants
Greater the value of ‘a’, stronger are the intermolecular forces of attraction and gas can be liquefied more easily.
It is related to incompressible volume of the molecules.
Units of ‘a’ and ’
Explanation of Behaviour of Real Gases
At very low pressures or at very high temperatures
Gases behave like ideal gases
At moderate pressures, for 1 mole of a gas
- At high pressures, for 1 mole of a gas
Exceptional behavior of
This is due to the fact that intermolecular forces of attraction in them are negligible.
Value of ’
Liquefaction of Gases :
A gas can be liquefied by decreasing the temperature or by increasing the pressure or by the combination of both.
Critical constants
Critical Temperature,
Temperature above which a gas cannot be liquefied howsoever high the pressure may be.
It the maximum temperature at which a substance can exist as liquid.
Critical Pressure,
Minimum pressure required to liquefy the gas at its critical temperature.
Critical Volume,
Volume occupied by one mole of a gas at its critical temperature and critical pressure.
Andrew’s Isotherms: Isotherms of
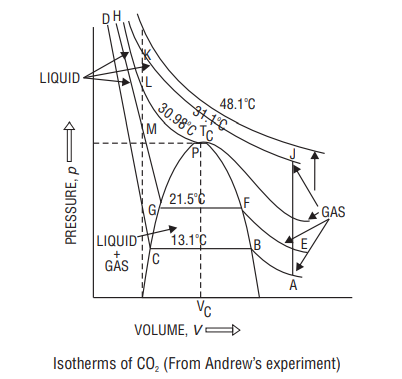
At
On increasing pressure, at B liquefaction starts.
After that, increase in pressure has little effect on volume, since liquids have low compressibility.
Above
Point
Difference between vapour and gas
Above critical temperature
Liquid State
Vapour Pressure
pressure exerted by the vapour present above the liquid in equilibrium with the liquid at that temperature.
Factors affecting vapour pressure
i. Nature of liquid - Weaker the intermolecular forces of attraction in a liquid, higher is the vapour pressure.
ii. Temperature-Vapour pressure increases with increase in temperature.
Normal boiling point-Boiling temperature under 1 atm pressure
Standard boiling point-Boiling temperature under 1 bar pressure
For
Standard boiling point
Surface Tension
The force acting per unit length perpendicular to the imaginary line drawn on the surface of liquid and towards the liquid side.
S.I. unit
Factors affecting surface tension
i. Nature of liquid - Stronger the intermolecular forces of attraction in a liquid, higher is the surface tension.
ii. Temperature-Surface tension decreases with increase in temperature.
Consequences of surface tension
i. Spherical shape of liquid drops - Surface tension tries to decrease the surface area of a liquid to minimum. Since for a given volume of liquid, sphere has the minimum surface area so liquid drops acquire spherical shape.
ii. Capillary action - Rise of liquid in capillary is due to the surface tension which pushes the liquid into the capillary tube.
Shape of Meniscus
Water has a concave meniscus whereas mercury has a convex meniscus : In case of water, adhesive forces are stronger than cohesive forces. In case of mercury, cohesive forces are stronger than adhesive forces.
Cohesive forces - Attractive forces existing between molecules of same substance e.g. between water molecules or mercury molecules.
Adhesive forces - Attractive forces existing between molecules of different substances like water and glass or mercury and glass.
Surface Energy
Energy required to increase the surface area of the liquid by one unit.
S.I. Unit
Dimensionally, surface tension and surface energy are same. Both have the same units.
Viscosity
A measure of friction / resistance to flow of liquid.
where,
S.I. units of
C.G.S unit of
1 Pas
Factors affecting viscosity
Nature of liquid - stronger the intermolecular forces of attraction, higher is the viscosity.
Temperature-viscosity decreases on increasing the temperature.
Solved Examples
Question 1. A sample of gas occupies
(a)
(b)
(c)
(d)
Show Answer
Solution: (c)
Since, Pressure is same
According to Charles law
Question 2. Pressure of a mixture of
(a)
(b)
(c)
(d)
Show Answer
Solution: (a)
According to the Dalton’s law of partial pressures
Question 3. A
(a)
(b)
(c)
(d)
Show Answer
Solution: (a)
1:1 mixture by weight of
For He:
For
According to Dalton’s law of partial pressure
Using,
Number of moles of
Volume
Therefore, concentration of
Question 4. A stockroom supervisor measured the contents of a partially filled
(a)
(b)
(c)
(d)
Show Answer
Solution: (c)
Vapour pressure of acetone remains unchanged at
Only the partial pressure of air (
Partial pressure of air (
Question 5. If
(a)
(b)
(c)
(d)
Show Answer
Solution: (a)
The value of volume used should be the volume of the container
Practice Questions
Question 1. If two moles of an ideal gas at
(a)
(b) 3 atm
(c)
(d)
Show Answer
Answer: (a)Question 2. The volume of a gas is
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)Question 3.
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)Question 4. Pure hydrogen sulphide is stored in a tank of 100 litre capacity at
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)Question 5. At
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)Question 6. Molecular weight of a gas that diffuses twice as rapidly as the gas with molecular weight 64 is
(a) 16
(b) 8
(c) 64
(d) 6.4
Show Answer
Answer: (a)Question 7. If rate of diffusion of
(a)
(b)
(c) 25
(d) 4
Show Answer
Answer: (a)Question 8. At what temperature, the rate of effusion of
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)Question 9. Equal weights of ethane and hydrogen are mixed in an empty container at
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)Question 10.
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)Question 11. If
(a) 100 Torr
(b) 650 Torr
(c) 1800 Torr
(d)
Show Answer
Answer: (b)Question 12. Which of the following is not the postulate of the kinetic theory of gases?
(a) Gas molecules are in a permanent state of random motion.
(b) Pressure of gas is due to molecular impacts on the walls.
(c) The molecules are perfectly elastic.
(d) The molecular collisions are elastic.
Show Answer
Answer: (c)Question 13. Maximum deviation from ideal gas is expected from
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)Question 14. The behavior of real gases approach ideal behavior at
(a) low temperature, low pressure
(b) high temperature, high pressure
(c) low temperature, high pressure
(d) high temperature, low pressure
Show Answer
Answer: (d)Question 15. At extremely low pressure the van der Waals equation for one mole of a gas may be written as:
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)Question 16. The value of van der Waals constant ’
(a) The pressure exerted by
(b) The pressure exerted by nitrogen is less than that of
(c) Both exertequal pressure
(d) None of these
Show Answer
Answer: (a)Question 17. Positive deviation from ideal gas behavior takes places because of
(a) Molecular interaction between atoms and
(b) Molecular interaction between atoms and
(c) Finite size of the atoms and
(d) Finite size of the atoms and
Show Answer
Answer: (a)Question 18. Consider the following plot
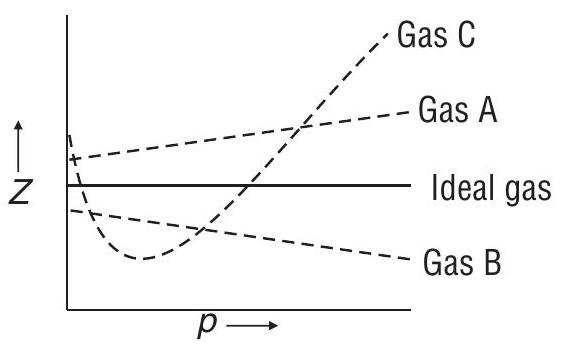
Which of the following statement is wrong?
(a) For a gas
(b) For gas
(c) Gas
(d) All van der Waals gases will behave like gas
Show Answer
Answer: (c)Question 19. Critical temperatures of
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)Question 20. The compressibility factor for a real gas a thigh pressure is
(a) 1
(b)
(c)
(d)
Show Answer
Answer: (b)Question 21. For one mole of a van der Waals gas when
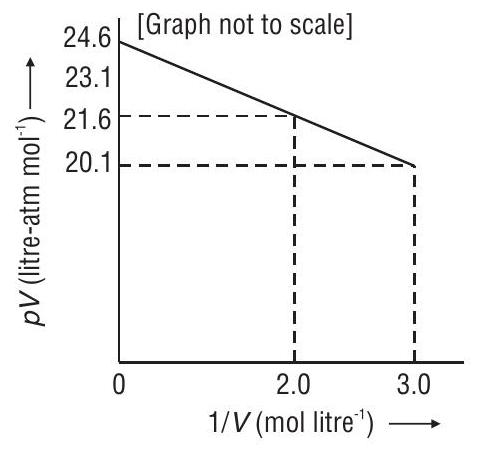
(a) 1.0
(b) 4.5
(c) 1.5
(d) 3.0
Show Answer
Answer: (c)Question 22. ‘a’ and ’
(a) aand
(b) aand
(c) afor
(d) afor
Show Answer
Answer: (d)Question 23. The compressibility of a gas is less than unity at NTP.
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)Question 24. A gas can be liquefied
(a) above its critical temperature
(b) at its critical temperature
(c) below its critical temperature
(d) at any temperature
Show Answer
Answer: (c)Question 25. On heating a liquid, its viscosity
(a) increases
(b) decreases
(c) remains same
(d) is reduced to zero
Show Answer
Answer: (b)Question 26. The surface tension of which of the following liquid is maximum?
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)Question 27. Choose the incorrect statement in the following:
(a) Surface tension is the force acting per unit length perpendicular to the line drawn on the surface of the liquid.
(b) Surface tension of a liquid increases with increase in temperature.
(c) The Sl unit of surface tension is
(d) Viscosity is a measure of resistance for the flow of liquid.
Show Answer
Answer: (b)Question 28. The gas with the highest critical temperature is
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)Question 29. Which of the following property of water be used to explain the spherical shape of rain droplets?
(a) viscosity
(b) surface phenomenon
(c) critical phenomena
(d) vapour pressure
Show Answer
Answer: (b)Question 30. The SI unit of viscosity coefficient
(a) Pascal
(b)
(c)
(d)
Show Answer
Answer: (b)Question 31. How will the viscosity of liquid be affected by the increase in temperature?
(a) Increase
(b) No effect
(c) Decrease
(d) No regular pattern will be followed
Show Answer
Answer: (c)Question 32. The van der Waals constant ’
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)Question 33. The incorrect statement among the following is
(a) The boiling point of a liquid at one bar is called standard boiling point of the liquid.
(b) The vapour pressure of a liquid is constant at constant temperature.
(c) The SI unit of coefficient of viscosity of a liquid is pascal second.
(d) The boiling point of a liquid is the same at all external pressures.
Show Answer
Answer: (d)Question 34. The van der Waals equation reduces itself to the ideal gas equation at:
(a) High pressure and low temperature
(b) Low pressure and low temperature
(c) low pressure and high temperature
(d) high pressure alone
Show Answer
Answer: (c)Question 35. The maximum number of molecules is present in :
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)Question 36. A certain sample of a gas has a volume of 0.2 litre measured at
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)Question 37. A gas deviates from ideal behavior at a high pressure because its molecules:
(a) have kinetic energy
(b) are bound by covalent bonds
(c) attract one another
(d) show the Tyndall effect
Show Answer
Answer: (c)Question 38. The pressure exerted by 1 mole of methane in a 0.25 litre container at
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)Question 39. To what temperature must a neon gas sample be heated to double its pressure, if the initial volume of a gas at
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)Question 40. Which pair of the gases diffuses with the same rate at same temperature and pressure?
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)Question 41. A gas has double the average velocity of
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)Question 42. The density of a gas is
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)Question 43. Non-ideal gases approach ideal behavior:
(a) high temperature and high pressure
(b) high temperature and low pressure
(c) low temperature and high pressure
(d) low temperature and low pressure
Show Answer
Answer: (b)Question 44. According to kinetic theory of gases for a diatomic molecule:
(a) the pressure exerted by the gas is proportional to the mean square speed of the molecules
(b) the pressure exerted by the gas is proportional to the root mean square speed of the molecules
(c) the root mean square speed is inversely proportional to the temperature
(d) the mean translational K.E. of the molecule, is directly proportional to the square root of the absolute temperature
Show Answer
Answer: (a)Question 45. The root mean square velocity of one mole of a monoatomic gas having molar mass
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)Question 46. The term that corrects for the attractive forces present in a real gas in the van der Waals equation is:
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)Question 47. What is the dominant intermolecular force or bond that must be overcome in converting liquid
(a) Dipole-dipole interaction
(b) Covalent bonds
(c) London-dispersion force
(d) Hydrogen bonding
Show Answer
Answer: (d)Question 48. Most probable speed, average speed and rms speed are related as:
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)Question 49. The rms speed of hydrogen molecules at room temperature is
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)Question 50. The critical temperature of a gas is that temperature:
(a) above which it can no longer remain in the gaseous state
(b) above which it cannot be liquefied by pressure
(c) at which itsolidifies
(d) at which the volume of the gas becomes zero
Show Answer
Answer: (b)Question 51. Two samples of gases
(a) 16
(b) 4
(c)
(d)
Show Answer
Answer: (d)Question 52. The compressibility factor of one mole of a gas is defined as
(a) zero
(b) 1
(c) -1
(d) infinity
Show Answer
Answer: (b)Question 53. A mixture of three gases
(a) X will be at the top of the vessel
(b) Ywill be at the top of the vessel
(c) Z will be at the top of the vessel
(d) will mix homogeneously throughout the vessel
Show Answer
Answer: (d)Question 54. When an ideal gas undergoes unrestricted expansion, no cooling occurs because the molecules:
(a) exert no attractive forces on each other
(b) do work equal to loss of kinetic energy
(c) collide without loss of energy
(d) are above the inversion temperature
Show Answer
Answer: (a)Question 55. Which of the following mixture of gases at room temperature would not follow Dalton’s law of partial pressures?
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)Question 56. The root mean square velocity of an ideal gas at constant pressure, varies with density (
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)Question 57. Equal masses of methane and oxygen are mixed in an empty container at
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)Question 58. When
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)Question 59. The molecular velocity of any gas is:
(a) directly proportional to square of temperature
(b) directly proportional to square root of temperature
(c) inversely proportional to square root of temperature
(d) inversely proportional to absolute temperature
Show Answer
Answer: (b)Question 60. Under critical state of a gas, for one mole of a gas, compressibility factor is :
(a)
(b) 1
(c)
(d)
Show Answer
Answer: (a)Question 61. A
(a) 300 Torr
(b) 150 Torr
(c) 66.66 Torr
(d) 133.3 Torr
Show Answer
Answer: (d)Question 62. The temperature at which
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)Question 63. A large irregularly - shaped closed tank is first evacuated and then connected to a 50 litre cylinder containing compressed nitrogen gas. The gas pressure in the cylinder, originally at 21.0 atm, falls to
(a) 475 litres
(b) 50 litres
(c) 525 litres
(d) 21.45 litres










