Unit 12 Aldehydes, Ketones And Carboxylic Acids (Intext Questions-4)
Intext Question
12.4 Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions.
(i) Ethanal, Propanal, Propanone, Butanone.
(ii) Benzaldehyde, $p$-Tolualdehyde, $p$-Nitrobenzaldehyde, Acetophenone.
Hint: Consider steric effect and electronic effect.
Show Answer
Answer

The + l effect of the alkyl group increases in the order:
Ethanal $<$ Propanal $<$ Propanone $<$ Butanone
The electron density at the carbonyl carbon increases with the increase in the $+I$ effect. As a result, the chances of attack by a nucleophile decrease. Hence, the increasing order of the reactivities of the given carbonyl compounds in nucleophilic addition reactions is:
Butanone $<$ Propanone $<$ Propanal $<$ Ethanal
(ii)

The $+l$ effect is more in ketone than in aldehyde. Hence, acetophenone is the least reactive in nucleophilic addition reactions. Among aldehydes, the $+l$ effect is the highest in $p$-tolualdehyde because of the presence of the electrondonating - $\mathrm{CH_3}$ group and the lowest in $p$-nitrobezaldehyde because of the presence of the electron-withdrawing $\mathrm{NO_2}$ group. Hence, the increasing order of the reactivities of the given compounds is:
Acetophenone $<p$-tolualdehyde $<$ Benzaldehyde
$<p$-Nitrobenzaldehyde
12.5 Predict the products of the following reactions:
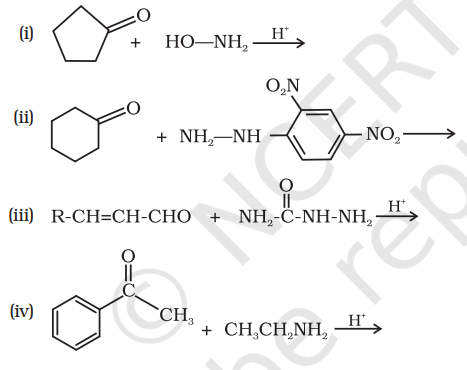
Show Answer
Answer
(i)

(ii)

(iii)

(iv)











