Unit 10 Haloalkanes And Haloarenes (Intext Questions-2)
Intext Questions
10.2 Why is sulphuric acid not used during the reaction of alcohols with KI?
Show Answer
Answer
In the presence of sulphuric acid $\left(\mathrm{H_2} \mathrm{SO_4}\right)$, $\mathrm{KI}$ produces $\mathrm{HI}$
$ 2 \mathrm{KI}+\mathrm{H_2} \mathrm{SO_4} \longrightarrow 2 \mathrm{KHSO_4}+2 \mathrm{HI} $
Since $\mathrm{H_2} \mathrm{SO_4}$ is an oxidizing agent, it oxidizes $\mathrm{HI}$ (produced in the reaction to $\mathrm{I_2}$.
$ 2 \mathrm{HI}+\mathrm{H_2} \mathrm{SO_4} \longrightarrow \mathrm{I_2}+\mathrm{SO_2}+\mathrm{H_2} \mathrm{O} $
As a result, the reaction between alcohol and $\mathrm{HI}$ to produce alkyl iodide cannot occur. Therefore, sulphuric acid is not used during the reaction of alcohols with KI. Instead, a non-oxidizing acid such as $\mathrm{H_3} \mathrm{PO_4}$ is used.
10.3 Write structures of different dihalogen derivatives of propane.
Show Answer
Answer
There are four different dihalogen derivatives of propane. The structures of these derivatives are shown below.
(i)
$\mathrm{Br - \underset{\substack{| \\ \mathrm{Br}}}{CH} - CH_2 -CH_3}$
1,1-Dibromopropane
(ii)
$\mathrm{CH_3 - \stackrel{\substack{\mathrm{Br} \\ |}}{\underset{\substack{ | \\ \mathrm{Br}}}{C}} -CH_3}$
2,2-Dibromopropane
(iii)
$\mathrm{Br -CH_3 - \stackrel{\substack{\mathrm{Br} \\ |}}{CH} - CH_3}$
1,2-Dibromopropane
(iv)
$\mathrm{Br - CH_2 -CH_2 -CH_2 -Br}$
1,3-Dibromopropane
10.4 Among the isomeric alkanes of molecular formula C5H12, identify the one that on photochemical chlorination yields (i) A single monochloride. (ii) Three isomeric monochlorides. (iii) Four isomeric monochlorides.
Show Answer
Answer
(i) To have a single monochloride, there should be only one type of $\mathrm{H}$-atom in the isomer of the alkane of the molecular formula $\mathrm{C_5} \mathrm{H_12}$. This is because, replacement of any $\mathrm{H}$-atom leads to the formation of the same product. The isomer is neopentane.
$\mathrm{CH_3 - \stackrel{\substack{\mathrm{CH_3} \\ |}}{\underset{\substack{ | \\ \mathrm{CH_3}}}{C}} -CH_3}$
Neopentane
Therefore, the isomer is $n$-pentane. It can be observed that there are three types of $\mathrm{H}$ atoms labelled as $a, b$ and $c$ in $n$-pentane.

(iii) To have four isomeric monochlorides, the isomer of the alkane of the molecular formula $\mathrm{C_5} \mathrm{H_12}$ should contain four different types of $\mathrm{H}$-atoms. Therefore, the isomer is 2-methylbutane. It can be observed that there are four types of $\mathrm{H}$ atoms labelled as $a, b, c$, and $d$ in 2-methylbutane.

10.5 Draw the structures of major monohalo products in each of the following reactions:
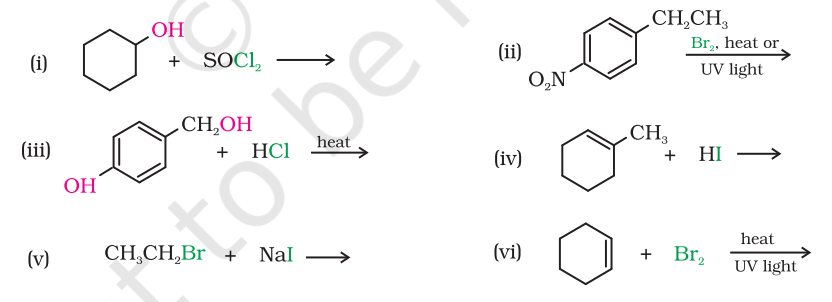
Show Answer
Answer
(i)

(ii)












