Unit 15 Polymers-Deleted
Do you think that daily life would have been easier and colourful without the discovery and varied applications of polymers? The use of polymers in the manufacture of plastic buckets, cups and saucers, children’s toys, packaging bags, synthetic clothing materials, automobile tyres, gears and seals, electrical insulating materials and machine parts has completely revolutionised the daily life as well as the industrial scenario. Indeed, the polymers are the backbone of four major industries viz. plastics, elastomers, fibres and paints and varnishes.
The word ‘polymer’ is coined from two Greek words: poly means many and mer means unit or part. The term polymer is defined as very large molecules having high molecular mass $\left(10^{3}-10^{7} \mathrm{u}\right)$. These are also referred to as macromolecules, which are formed by joining of repeating structural units on a large scale. The repeating structural units are derived from some simple and reactive molecules known as monomers and are linked to each other by covalent bonds. The process of formation of polymers from respective monomers is called polymerisation.
15.1 Classification of Polymers
There are several ways of classification of polymers based on some special considerations. One of the common classifications of polymers is based on source from which polymer is derived.
Under this type of classification, there are three sub categories.
1. Natural polymers
These polymers are found in plants and animals. Examples are proteins, cellulose, starch, some resins and rubber.
2. Semi-synthetic polymers
Cellulose derivatives as cellulose acetate (rayon) and cellulose nitrate, etc. are the usual examples of this sub category.
3. Synthetic polymers
A variety of synthetic polymers as plastic (polythene), synthetic fibres (nylon 6,6) and synthetic rubbers (Buna - S) are examples of man-made polymers extensively used in daily life as well as in industry.
Polymers can also be classified on the basis of their structure, molecular forces or modes of polymerisation.
Intext Questions
15.1 What are polymers?
Show Answer
Answer
Polymers are high molecular mass macromolecules, which consist of repeating structural units derived from monomers. Polymers have a high molecular mass $\left(10^{3}-10^{7} u\right)$. In a polymer, various monomer units are joined by strong covalent bonds. These polymers can be natural as well as synthetic. Polythene, rubber, and nylon 6, 6 are examples of polymers.
15.2 Types of Polymerisation Reactions
There are two broad types of polymerisation reactions, i.e., the addition or chain growth polymerisation and condensation or step growth polymerisation.
15.2.1 Addition Polymerisation or Chain Growth Polymerisation
In this type of polymerisation, the molecules of the same monomer or diferent monomers add together on a large scale to form a polymer. The monomers used are unsaturated compounds, e.g., alkenes, alkadienes and their derivatives. This mode of polymerisation leads to an increase in chain length and chain growth can take place through the formation of either free radicals or ionic species. However, the free radical governed addition or chain growth polymerisation is the most common mode.
15.2.1.1 Mechanism of Addition Polymerisation
1. Free radical mechanism
A variety of alkenes or dienes and their derivatives are polymerised in the presence of a free radical generating initiator (catalyst) like benzoyl peroxide, acetyl peroxide, tert-butyl peroxide, etc. For example, the polymerisation of ethene to polythene consists of heating or exposing to light a mixture of ethene with a small amount of benzoyl peroxide initiator. The process starts with the addition of phenyl free radical formed by the peroxide to the ethene double bond thus generating a new and larger free radical. This step is called chain initiating step. As this radical reacts with another molecule of ethene, another bigger sized radical is formed. The repetition of this sequence with new and bigger radicals carries the reaction forward and the step is termed as chain propagating step. Ultimately, at some stage the product radical thus formed reacts with another radical to form the polymerised product. This step is called the chain terminating step. The sequence of steps involved in the formation of polythene are depicted as follows:
Chain initiation steps
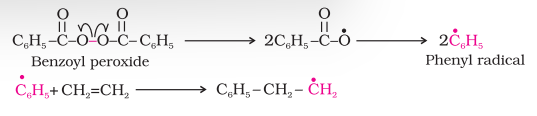
Chain propagation
$$ \begin{aligned} \mathrm{C_6} \mathrm{H_5}-\mathrm{CH_2}-\dot{\mathrm{C}} \mathrm{H_2}+\mathrm{CH_2}=\mathrm{CH_2} \longrightarrow & \mathrm{C_6} \mathrm{H_5}-\mathrm{CH_2}-\mathrm{CH_2}-\mathrm{CH_2}-\dot{\mathrm{C}} \mathrm{H_2} \\ & \\ & \mathrm{C_6} \mathrm{H_5}+\mathrm{CH_2}-\mathrm{CH_2}+{ _\mathrm{n}} \mathrm{CH_2}-\dot{\mathrm{C}} \mathrm{H_2} \end{aligned} $$
Chain terminating step
For termination of the long chain, these free radicals can combine in different ways to form polythene. One mode of termination of chain is shown as under:
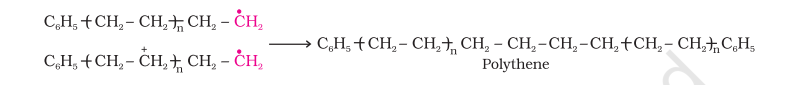
The addition polymers formed by the polymerisation of a single monomeric species are known as homopolymers, for example polythene discussed above is a homopolymer.

The polymers made by addition polymerisation from two different monomers are termed as copolymers. Buna-S, which is formed by polymerisation of buta–1, 3–diene and styrene is an example of copolymer formed by addition polymerisation.
15.2.1.2 Some Important Addition Polymers
(a) Polythene
Polythenes are linear or slightly branched long chain molecules. These are capable of repeatedly softening on heating and hardening on cooling and are thus thermoplastic polymers. There are two types of polythene as given below:
(i) Low density polythene: It is obtained by the polymerisation of ethene under high pressure of 1000 to 2000 atmospheres at a temperature of $350 \mathrm{~K}$ to $570 \mathrm{~K}$ in the presence of traces of dioxygen or a peroxide initiator (catalyst). The low density polythene (LDP) is obtained through the free radical addition and $\mathrm{H}$-atom abstraction. It has highly branched structure. These polymers have straight chain structure with some branches as shown below.
Low density polythene is chemically inert and tough but flexible and a poor conductor of electricity. Hence, it is used in the insulation of electricity carrying wires and manufacture of squeeze bottles, toys and flexible pipes.
(ii) High density polythene: It is formed when addition polymerisation of ethene takes place in a hydrocarbon solvent in the presence of a catalyst such as triethylaluminium and titanium tetrachloride (Ziegler-Natta catalyst) at a temperature of $333 \mathrm{~K}$ to $343 \mathrm{~K}$ and under a pressure of 6-7 atmospheres. High density polythene (HDP) thus produced, consists of linear molecules as shown below and has a high density due to close packing. Such polymers are also called linear polymers. High density polymers are also chemically inert and more tough and hard. It is used for manufacturing buckets, dustbins, bottles, pipes, etc.

( b )Polytetrafluoroethene (Teflon) Teflon is manufactured by heating tetrafluoroethene with a free radical or persulphate catalyst at high pressures. It is chemically inert and resistant to attack by corrosive reagents. It is used in making oil seals and gaskets and also used for non - stick surface coated utensils.

(c) Polyacrylonitrile The addition polymerisation of acrylonitrile in presence of a peroxide catalyst leads to the formation of polyacrylonitrile.
$$ \underset{\text { Tetrafluoroethene }}{\mathrm{nCC_{2 }}=\mathrm{CF_2}} \xrightarrow[\text { High pressure }]{\text { Catalyst }} \underset{\text { Teflon }}{\left[\mathrm{CF_2}-\mathrm{CF_2}\right]_{\mathrm{n}}} $$
Polyacrylonitrile is used as a substitute for wool in making commercial fibres as orlon or acrilan.
Example 15.1
Is $+\mathrm{CH_2}-\mathrm{CH}\left(\mathrm{C_6} \mathrm{H_5}\right)+_{n}$ a homopolymer or a copolymer?
Solution
It is a homopolymer and the monomer from which it is obtained is styrene $\mathrm{C_6} \mathrm{H_5} \mathrm{CH}=\mathrm{CH_2}$.
15.2.2 Condensation Polymerisation or Step Growth Polymerisation
This type of polymerisation generally involves a repetitive condensation reaction between two bi-functional or trifunctional mono-meric units. These polycondensation reactions may result in the loss of some simple molecules as water, alcohol, hydrogen chloride, etc., and lead to the formation of high molecular mass condensation polymers.
In these reactions, the product of each step is again a bi-functional species and the sequence of condensation goes on. Since, each step produces a distinct functionalised species and is independent of each other, this process is also called as step growth polymerisation.
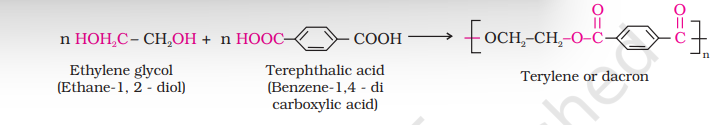
The formation of terylene or dacron by the interaction of ethylene glycol and terephthalic acid is an example of this type of polymerisation.
15.2.2.1 Some Important Condensation Polymers
(a) Polyamides
These polymers possessing amide linkages are important examples of synthetic fibres and are termed as nylons. The general method of preparation consists of the condensation polymerisation of diamines with dicarboxylic acids or condensation of amino acids or their lactams.
Nylons
(i) Nylon 6,6: It is prepared by the condensation polymerisation of hexamethylenediamine with adipic acid under high pressure and at high temperature.
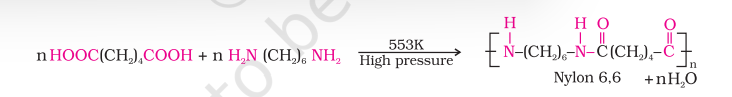
Nylon 6, 6 is fibre forming solid. It possess high tensile strength. This characteristic can be attributed to the strong intermolecular forces like hydrogen bonding. These strong forces also lead to close packing of chains and thus impart crystalline nature.
Nylon 6, 6 is used in making sheets, bristles for brushes and in textile industry.
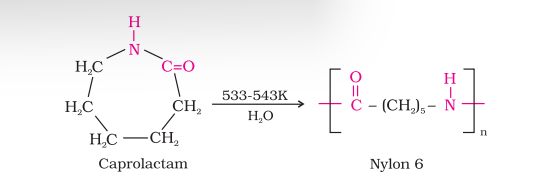
(ii) Nylon 6: It is obtained by heating caprolactum with water at a high temperature. Nylon 6 is used for the manufacture of tyre cords, fabrics and ropes.
(b) Polyesters
These are the polycondensation products of dicarboxylic acids and diols. Dacron or terylene is the best known example of polyesters. It is manufactured by heating a mixture of ethylene glycol and terephthalic acid at 420 to $460 \mathrm{~K}$ in the presence of zinc acetate-antimony trioxide catalyst as per the reaction given earlier. Dacron fibre (terylene) is crease resistant and is used in blending with cotton and wool fibres and also as glass reinforcing materials in safety helmets, etc.
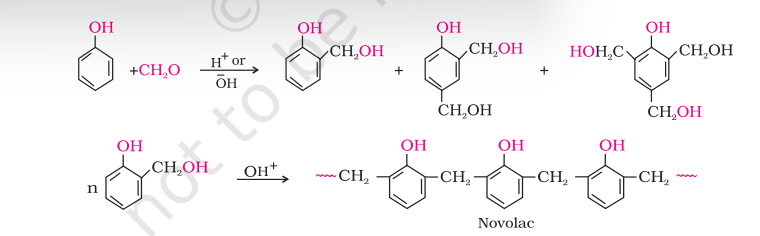
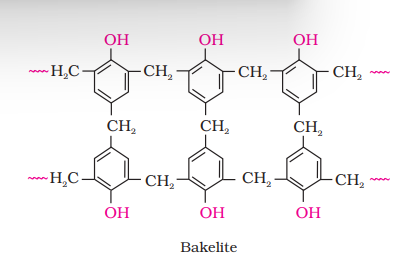
(c) Phenol - formaldehyde polymer (Bakelite and related polymers)
Phenol - formaldehyde polymers are the oldest synthetic polymers. These are obtained by the condensation reaction of phenol with formaldehyde in the presence of either an acid or a base catalyst. The reaction starts with the initial formation of $o$-and/or $p$-hydroxymethylphenol derivatives, which further react with phenol to form compounds having rings joined to each other through- $\mathrm{CH_2}$ groups. The initial product could be a linear product - Novolac used in paints.
Novolac on heating with formaldehyde undergoes cross linking to form an infusible solid mass called bakelite. It is thermosetting polymer which cannot be reused or remoulded. Thus, bakelite is formed by cross linking of linear chains of the polymer novolac. Bakelite is used for making combs, phonograph records, electrical switches and handles of various utensils.
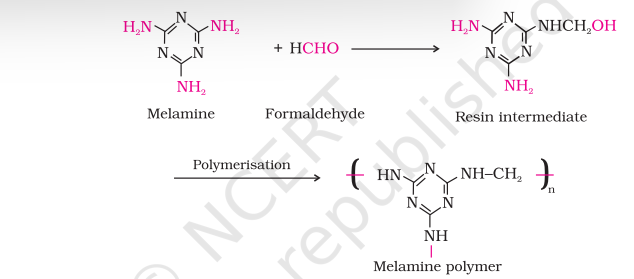
(d) Melamine — formaldehyde polymer
Melamine formaldehyde polymer is formed by the condensation polymerisation of melamine and formaldehyde.
Intext Questions
15.2 Write the names of monomers of the following polymers
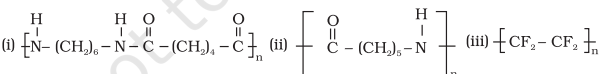
Answer (i) Hexamethylenediamine $\left[\mathrm{H _2} \mathrm{~N}-\left(\mathrm{CH_2}\right) _{6}-\mathrm{NH _2}\right] \text {and adipic acid } \left[\mathrm{HOOC}-\left(\mathrm{CH _2}\right) _{4}-\mathrm{COOH}\right]$ (ii) Caprolactam (iii) Tetrafluoroethene $\left(\mathrm{CF_2}=\mathrm{CF_2}\right)$Show Answer

Show Answer
Answer
Addition polymers:
Polyvinyl chloride, polythene
Condensation polymers:
Terylene, bakelite
15.2.3 Copolymerisation
Copolymerisation is a polymerisation reaction in which a mixture of more than one monomeric species is allowed to polymerise and form a copolymer. The copolymer can be made not only by chain growth polymerisation but by step growth polymerisation also. It contains multiple units of each monomer used in the same polymeric chain.
For example, a mixture of buta–1, 3–diene and styrene can form a copolymer.
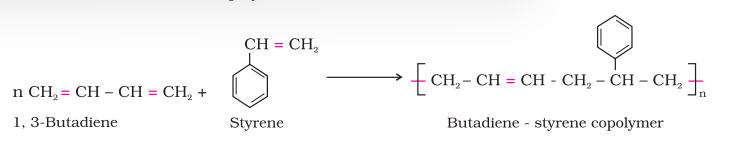
Copolymers have properties quite different from homopolymers. For example, butadiene - styrene copolymer is quite tough and is a good substitute for natural rubber. It is used for the manufacture of autotyres, floortiles, footwear components, cable insulation, etc.
15.2.4 Rubber
1. Natural rubber
Rubber is a natural polymer and possesses elastic properties. It is also termed as elastomeric polymer. In elastomeric polymers, the polymer chains are held together by the weak intermolecular forces. These weak binding forces permit the polymer to be stretched. A few ‘crosslinks’ are introduced in between the chains, which help the polymer to retract to its original position after the force is released.
Rubber has a variety of uses. It is manufactured from rubber latex which is a colloidal dispersion of rubber in water. This latex is obtained from the rubber tree which is found in India, Srilanka, Indonesia, Malaysia and South America.
Natural rubber may be considered as a linear polymer of isoprene (2-methyl-1, 3-butadiene) and is also called as cis - 1, 4 - polyisoprene.
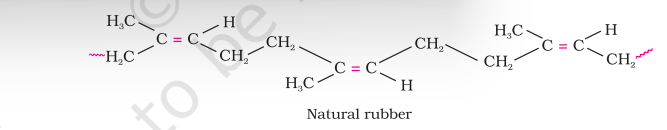
The cis-polyisoprene molecule consists of various chains held together by weak van der Waals interactions and has a coiled structure. Thus, it can be stretched like a spring and exhibits elastic properties.
Vulcanisation of rubber: Natural rubber becomes soft at high temperature ( $>335 \mathrm{~K}$ ) and brittle at low temperatures (<283 $\mathrm{K}$) and shows high water absorption capacity. It is soluble in nonpolar solvents and is non-resistant to attack by oxidising agents. To improve upon these physical properties, a process of vulcanisation is carried out. This process consists of heating a mixture of raw rubber with sulphur and an appropriate additive at a temperature range between $373 \mathrm{~K}$ to $415 \mathrm{~K}$. On vulcanisation, sulphur forms cross links at the reactive sites of double bonds and thus the rubber gets stiffened.
In the manufacture of tyre rubber, 5% of sulphur is used as a crosslinking agent. The probable structures of vulcanised rubber molecules are depicted below:
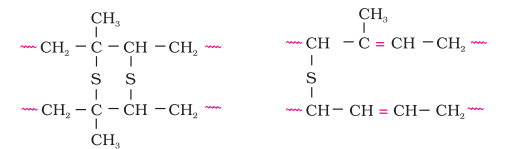
2. Synthetic rubbers
Synthetic rubber is any vulcanisable rubber like polymer, which is capable of getting stretched to twice its length. However, it returns to its original shape and size as soon as the external stretching force is released. Thus, synthetic rubbers are either homopolymers of 1, 3 - butadiene derivatives or copolymers of 1, 3 - butadiene or its derivatives with another unsaturated monomer.
Preparation of Synthetic Rubbers
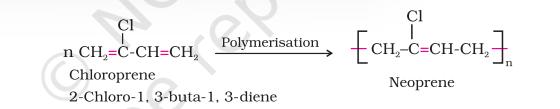
1. Neoprene
Neoprene or polychloroprene is formed by the free radical polymerisation of chloroprene.
It has superior resistance to vegetable and mineral oils. It is used for manufacturing conveyor belts, gaskets and hoses.
2. Buna - N
You have already studied about Buna-S, in Section 15.1.3. Buna $-\mathrm{N}$ is obtained by the copolymerisation of 1,3 - buta-1, 3-diene and acrylonitrile in the presence of a peroxide catalyst.

It is resistant to the action of petrol, lubricating oil and organic solvents. It is used in making oil seals, tank lining, etc.
Intext Questions
15.4 Explain the difference between Buna-N and Buna-S.
Answer Buna - $\mathrm{N}$ is a copolymer of 1,3 -butadiene and acrylonitrile. Buna - $\mathrm{S}$ is a copolymer of 1 , 3-butadiene and styrene.Show Answer
(i) Nylon 6, 6, Buna-S, Polythene.
(ii) Nylon 6, Neoprene, Polyvinyl chloride.
Show Answer
Answer
Different types of polymers have different intermolecular forces of attraction. Elastomers or rubbers have the weakest while fibres have the strongest intermolecular forces of attraction. Plastics have intermediate intermolecular forces of attraction. Hence, the increasing order of the intermolecular forces of the given polymers is as follows:
(i) Buna - S < polythene < Nylon 6, 6
(ii) Neoprene < polyvinyl chloride < Nylon 6
15.3 Molecular Mass of Polymers
Polymer properties are closely related to their molecular mass, size and structure. The growth of the polymer chain during their synthesis is dependent upon the availability of the monomers in the reaction mixture. Thus, the polymer sample contains chains of varying lengths and hence its molecular mass is always expressed as an average. The molecular mass of polymers can be determined by chemical and physical methods.
15.4 Biodegradable Polymers
A large number of polymers are quite resistant to the environmental degradation processes and are thus responsible for the accumulation of polymeric solid waste materials. These solid wastes cause acute environmental problems and remain undegraded for quite a long time. In view of the general awareness and concern for the problems created by the polymeric solid wastes, certain new biodegradable synthetic polymers have been designed and developed. These polymers contain functional groups similar to the functional groups present in biopolymers.
Aliphatic polyesters are one of the important classes of biodegradable polymers. Some important examples are given below:
1. Poly $\beta$-hydroxybutyrate - co- $\beta$-hydroxy valerate (PHBV)
It is obtained by the copolymerisation of 3-hydroxybutanoic acid and 3 - hydroxypentanoic acid. PHBV is used in speciality packaging, orthopaedic devices and in controlled release of drugs. PHBV undergoes bacterial degradation in the environment.
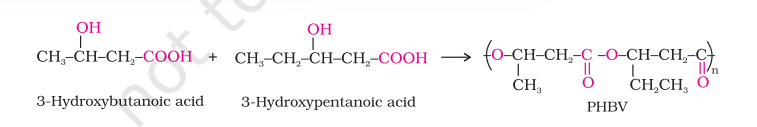
2. Nylon 2–nylon 6
It is an alternating polyamide copolymer of glycine $\left(\mathrm{H_2} \mathrm{~N}-\mathrm{CH_2}-\right.$ $\mathrm{COOH})$ and amino caproic acid $\left[\mathrm{H_2} \mathrm{~N}\left(\mathrm{CH_2}\right)_{5} \mathrm{COOH}\right]$ and is biodegradable. Can you write the structure of this copolymer?
15.5 Polymers of Commercial Importance
Besides, the polymers already discussed, some other commercially important polymers along with their structures and uses are given below in Table 15.1.
Table 15.1: Some Other Commercially Important Polymers
| Name of Polymer | Monomer | Structure | Uses |
|---|---|---|---|
| Polypropene | Propene | Manufacture of ropes, toys, pipes, fibres, etc. |
|
| Polystyrene | Styrene | As insulator, wrapping material, manufacture of toys, radio and television cabinets. |
|
| Polyvinyl chloride (PVC) |
Vinyl chloride | Manufacture of rain coats, hand bags, vinyl flooring, water pipes. |
|
| Urea-formaldehyle Resin |
(a) Urea (b) Formaldehyde |
For making unbreak- able cups and laminated sheets. |
|
| Glyptal | (a) Ethylene glycol (b) Phthalic acid |
Manufacture of paints and lacquers. |
|
| Bakelite | (a) Phenol (b) Formaldehyde |
For making combs, electrical switches, handles of utensils and computer discs. |
Summary
Polymers are defined as high molecular mass macromolecules, which consist of repeating structural units derived from the corresponding monomers. These polymers may be of natural or synthetic origin and are classified in a number of ways.
In the presence of an organic peroxide initiator, the alkenes and their derivatives undergo addition polymerisation or chain growth polymerisation through a free radical mechanism. Polythene, teflon, orlon, etc. are formed by addition polymerisation of an appropriate alkene or its derivative. Condensation polymerisation reactions are shown by the interaction of bi - or poly functional monomers containing - NH2, - OH and - COOH groups. This type of polymerisation proceeds through the elimination of certain simple molecules as H2O, CH3OH, etc. Formaldehyde reacts with phenol and melamine to form the corresponding condensation polymer products. The condensation polymerisation progresses through step by step and is also called as step growth polymerisation. Nylon, bakelite and dacron are some of the important examples of condensation polymers. However, a mixture of two unsaturated monomers exhibits copolymerisation and forms a co-polymer containing multiple units of each monomer. Natural rubber is a cis 1, 4-polyisoprene and can be made more tough by the processof vulcanisation with sulphur. Synthetic rubbers are usually obtained by copolymerisation of alkene and 1, 3 butadiene derivatives.
In view of the potential environmental hazards of synthetic polymeric wastes, certain biodegradable polymers such as PHBV and Nylon-2- Nylon-6 are developed as alternatives.
Exercises
15.1 Explain the terms polymer and monomer.
Answer Polymers are high molecular mass macromolecules composed of repeating structural units derived from monomers. Polymers have a high molecular mass $\left(10^{3}-10^{7} \mathrm{u}\right)$. In a polymer, various monomer units are joined by strong covalent bonds. Polymers can be natural as well as synthetic. Polythene, rubber, and nylon 6,6 are examples of polymers. Monomers are simple, reactive molecules that combine with each other in large numbers through covalent bonds to give rise to polymers. For example, ethene, propene, styrene, vinyl chloride.Show Answer
Answer Natural polymers are polymers that are found in nature. They are formed by plants and animals. Examples include protein, cellulose, starch, etc. Synthetic polymers are polymers made by human beings. Examples include plastic (polythene), synthetic fibres (nylon 6, 6), synthetic rubbers (Buna - S).Show Answer
AnswerShow Answer
Homopolymer
Copolymer
The polymers that are formed by the polymerization of a single monomer are known as homopolymers. In other words, the repeating units of homopolymers are derived only from one monomer. For example, polythene is a homopolymer of ethene.
The polymers whose repeating units are derived from two types of monomers are known as copolymers. For example, Buna - S is a copolymer of 1, 3-butadiene and styrene.
Answer The functionality of a monomer is the number of binding sites that is/are present in that monomer. For example, the functionality of monomers such as ethene and propene is one and that of 1,3-butadiene and adipic acid is two.Show Answer
Answer Polymerization is the process of forming high molecular mass $\left(10^{3}-10^{7} \mathrm{u}\right)$ macromolecules, which consist of repeating structural units derived from monomers. In a polymer, various monomer units are joined by strong covalent bonds.Show Answer
Answer $(\mathrm{NH}-\mathrm{CHR}-\mathrm{CO})_{n}$ is a homopolymer because it is obtained from a single monomer unit, $\mathrm{NH_2}-\mathrm{CHR}-\mathrm{COOH}$.Show Answer
Answer Elastic properties in elastomers: Elastomers possess elastic properties because the intermolecular forces are the weakest. As a result, they can be readily stretched by applying small stress and regain their original shape when the stress is removed. The elasticity can be further increased by introducing some cross - links in the polymer chains. Natural rubber is the most popular example of elastomers. A few more examples are buna-S, buna- $\mathrm{N}$ and neoprene.Show Answer
Answer Addition polymerization is the process of repeated addition of monomers, possessing double or triple bonds to form polymers. For example, polythene is formed by addition polymerization of ethene. $\left.n \mathrm{CH_2}=\mathrm{CH_2} \longrightarrow (\mathrm{CH_2}-\mathrm{CH_2}\right)_{n}$ Ethene Polyethene Condensation polymerization is the process of formation of polymers by repeated condensation reactions between two different bi-functional or tri-functional monomers. A small molecule such as water or hydrochloric acid is eliminated in each condensation. For example, nylon 6, 6 is formed by condensation polymerization of hexamethylenediamine and adipic acid.Show Answer

Answer The process of forming polymers from two or more different monomeric units is called copolymerization. Multiple units of each monomer are present in a copolymer. The process of forming polymer Buna-S from 1, 3-butadiene and styrene is an example of copolymerization Nylon 6, 6 is also a copolymer formed by hexamethylenediamine and adipic acid.Show Answer


Answer Mechanism of free radical polymerization of ethene: Step I. Chain initiation step Step II. Chain propogation step Step III. Chain terminating step For termination of the long chain, these free radicals can combine in different ways to form polythene.Show Answer



Answer Thermoplastic polymers are linear (slightly branched) long chain polymers, which can be repeatedly softened and hardened on heating. Hence, they can be modified again and again. Examples include polythene, polystyrene. Thermosetting polymers are cross-linked or heavily branched polymers which get hardened during the molding process. These plastics cannot be softened again on heating. Examples of thermosetting plastics include bakelite, urea-formaldehyde resins.Show Answer
(i) Polyvinyl chloride
(ii) Teflon
(iii) Bakelite
Answer (i) Vinyl chloride $\left(\mathrm{CH_2}=\mathrm{CHCl}\right)$ (ii) Tetrafluoroethylene $\left(\mathrm{CF_2}=\mathrm{CF_2}\right)$ (iii) Formaldehyde $(\mathrm{HCHO})$ and phenol $\left(\mathrm{C_6} \mathrm{H_5} \mathrm{OH}\right)$Show Answer
Answer One common initiator used in free radical addition polymerization is benzoyl peroxide. Its structure is given below.Show Answer

Answer Natural rubber is a linear cis-polyisoprene in which the double bonds are present between $C_{2}$ and $C_{3}$ of the isoprene units. Because of this cis-configuration, intermolecular interactions between the various strands of isoprene are quite weak. As a result, various strands in natural rubber are arranged randomly. Hence, it shows elasticity.Show Answer

Answer Natural rubber though useful has some problems associated with its use. These limitations are discussed below: 1. Natural rubber is quite soft and sticky at room temperature. At elevated temperatures ( $>335 \mathrm{~K}$ ), it becomes even softer. At low temperatures ( $<283 \mathrm{~K}$ ), it becomes brittle. Thus, to maintain its elasticity, natural rubber is generally used in the temperature range of $283 \mathrm{~K}-335 \mathrm{~K}$. 2. It has the capacity to absorb large amounts of water. 3. It has low tensile strength and low resistance to abrasion. 4. It is soluble in non-polar solvents. 5. It is easily attacked by oxidizing agents. Vulcanization of natural rubber is done to improve upon all these properties. In this process, a mixture of raw rubber with sulphur and appropriate additive is heated at a temperature range between $373 \mathrm{~K}$ and $415 \mathrm{~K}$.Show Answer
Answer The monomeric repeating unit of nylon 6 is $\left[\mathrm{NH}-\left(\mathrm{CH_2}\right)_{5}-\mathrm{CO}\right]$, which is derived from Caprolactam. The monomeric repeating unit of nylon 6,6 is $\left[\mathrm{NH}-\left(\mathrm{CH _2}\right) _{6}-\mathrm{NH}-\mathrm{CO}-\left(\mathrm{CH _2}\right) _{4}-\mathrm{CO}\right]$, which is derived from hexamethylene diamine and adipic acid.Show Answer
(i) Buna-S
(ii) Buna-N
(iii) Dacron
(iv) Neoprene
AnswerShow Answer
Polymer
Monomer
Structure of monomer
i
Buna-S
1,3 -butadiene
$\mathrm{CH_2}=\mathrm{CH}-\mathrm{CH}=\mathrm{CH_2}$
Styrene
$\mathrm{C_6} \mathrm{H_5} \mathrm{CH}=\mathrm{CH} 2$
ii
Buna-N
1, 3-butadiene
$\mathrm{CH_2}=\mathrm{CH}-\mathrm{CH}=\mathrm{CH_2}$
Acrylonitrile
$\mathrm{CH_2}=\mathrm{CH}-\mathrm{CN}$
iii
Neoprene
Chloroprene
$\mathrm{CH _2}=\stackrel{\substack{\mathrm{O} \\ |}}{\mathrm{C}}-\mathrm{CH}=\mathrm{CH _2}$
iv
Dacron
Ethylene glycol
$\mathrm{HOH_2} \mathrm{C}-\mathrm{CH_2} \mathrm{OH}$
Terephthalic acid

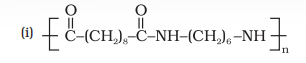
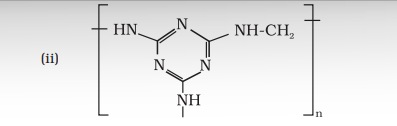
Answer (i) The monomers of the given polymeric structure are decanoic acid $\left[\mathrm{HOOC}-\left(\mathrm{CH _2}\right) _{8}-\mathrm{COOH}\right]$ and hexamethylene diamine $\left[\mathrm{H _2} \mathrm{~N}\left(\mathrm{CH _2}\right) _{6} \mathrm{NH _2}\right]$. (ii) The monomers of the given polymeric structure areShow Answer

Answer The condensation polymerisation of ethylene glycol and terephthalic acid leads to the formation of dacron.Show Answer

Show Answer
Answer
A polymer that can be decomposed by bacteria is called a biodegradable polymer.
Poly- $\beta$-hydroxybutyrate-CO- $\beta$ - hydroxyvalerate (PHBV) is a biodegradable aliphatic polyester.











