Chapter 06 Thermodynamics
Multiple Choice Questions (MCQs)
1. Thermodynamics is not concerned about
(a) energy changes involved in a chemical reaction
(b) the extent to which a chemical reaction proceeds
(c) the rate at which a reaction proceeds
(d) the feasibility of a chemical reaction
Show Answer
Answer
(c) Thermodynamics is not concerned with the rate at which a reaction proceeds. Thermodynamics deals with the energy change, feasibility and extent of a reaction, but not with the rate and mechanism of a process.
- (a) Thermodynamics is concerned with energy changes involved in a chemical reaction because it studies how energy is transferred and transformed during chemical processes.
- (b) Thermodynamics is concerned with the extent to which a chemical reaction proceeds because it involves the study of equilibrium states and how far a reaction will go before reaching equilibrium.
- (d) Thermodynamics is concerned with the feasibility of a chemical reaction because it determines whether a reaction can occur spontaneously based on the changes in enthalpy, entropy, and free energy.
2. Which of the following statement is correct?
(a) The presence of reacting species in a covered beaker is an example of open system.
(b) There is an exchange of energy as well as matter between the system and the surroundings in a closed system.
(c) The presence of reactants in a closed vessel made up of copper is an example of a closed system.
(d) The presence of reactants in a thermos flask or any other closed insulated vessel is an example of a closed system.
Show Answer
Answer
(c) For a closed vessel made of copper, no matter can exchange between the system and the surroundings but energy exchange can occur through its walls.
Presence of reaction species in a covered beaker-closed system and exchange of matter as well as energy-open-system. Presence of reactant in a closed vessel closed system and presence of reactant in thermos flask-isolated system.
-
(a) The presence of reacting species in a covered beaker is not an example of an open system because a covered beaker restricts the exchange of matter with the surroundings, making it a closed system.
-
(b) There is no exchange of matter between the system and the surroundings in a closed system; only energy exchange can occur. Therefore, the statement is incorrect.
-
(d) The presence of reactants in a thermos flask or any other closed insulated vessel is an example of an isolated system, not a closed system, because it prevents the exchange of both matter and energy with the surroundings.
3. The state of a gas can be described by quoting the relationship between
(a) pressure, volume, temperature
(b) temperature, amount, pressure
(c) amount, volume, temperature
(d) pressure, volume, temperature, amount
Show Answer
Answer
(d) The state of a gas can be described by quoting the relationship between pressure, volume, temperature and amount. The ideal gas equation is
Thus,
-
(a) pressure, volume, temperature: This option is incorrect because it does not include the amount of gas (n), which is a crucial variable in describing the state of a gas. The ideal gas law ( pV = nRT ) requires the amount of gas to fully describe the state.
-
(b) temperature, amount, pressure: This option is incorrect because it does not include the volume (V) of the gas. The volume is necessary to describe the state of the gas according to the ideal gas law ( pV = nRT ).
-
(c) amount, volume, temperature: This option is incorrect because it does not include the pressure (p) of the gas. Pressure is a key variable in the ideal gas law ( pV = nRT ) and is needed to fully describe the state of the gas.
4. The volume of gas is reduced to half from its original volume. The specific heat will be
(a) reduce to half
(b) be doubled
(c) remain constant
(d) increase four times
Show Answer
Answer
(c) The volume of gas is reduced to half from its original volume. The specific heat will be remain constant.
Specific heat is an intensive property depending only on the nature of the gas.
-
(a) The specific heat does not reduce to half because specific heat is an intensive property, meaning it does not depend on the amount of substance or its volume.
-
(b) The specific heat does not double because it is an intrinsic property of the material and remains constant regardless of changes in volume or mass.
-
(d) The specific heat does not increase four times because it is independent of the volume of the gas and is determined by the nature of the gas itself.
5. During complete combustion of one mole of butane,
(a)
(b)
(c)
(d)
Show Answer
Answer
(c) Given that, the complete combustion of one mole of butane is represented by thermochemical reaction as
We have to take the combustion of one mole of
-
Option (a) is incorrect because it represents the combustion of 2 moles of butane, not 1 mole. Additionally, the enthalpy change should be for 1 mole of butane, not 2 moles.
-
Option (b) is incorrect because the enthalpy change value is halved to
-
Option (d) is incorrect because the enthalpy change is given as positive (
6.
(a) zero
(b)
(c)
(d) equal to
Show Answer
Answer
(b) The reaction is
-
(a) Zero: The enthalpy change of formation,
-
(c)
-
(d) Equal to
7. In an adiabatic process, no transfer of heat takes place between system and surroundings. Choose the correct option for free expansion of an ideal gas under adiabatic condition from the following.
(a)
(b)
(c)
(d)
Show Answer
Answer
(c) Free expansion,
Adiabatic process,
Hence, when such a gas expands under adiabatic conditions into a vacuum, no heat is absorbed or evolved since no external work is done to separate the molecules.
-
Option (a): This option states that
-
Option (b): This option states that
-
Option (d): This option states that
8. The pressure-volume work for an ideal gas can be calculated by using the expression
(a)
(b)
(c)
(d)
Show Answer
Answer
(b) The correct option is W (reversible)


-
Option (a)
-
Option (c)
-
Option (d)
9. The entropy change can be calculated by using the expression
(a)
(b)
(c)
(d)
Show Answer
Answer
(c) The entropy change can be calculated by using the expression
When water freezes in a glass beaker,
Hence, entropy of the surrounding increases.
-
(a)
-
(b)
-
(d)
10. 0n the basis of theromochemical equations (1), (2) and (3), find out which of the algebraic relationships given in options (a) to (d) is correct
(a)
(b)
(c)
(d)
Show Answer
Answer
(c) The algebraic relationships of the given reaction is equation (a) -equation (b) = equation (c)
(a)
(b)
Hence,
-
Option (a)
-
Option (b)
-
Option (d)
11. Consider the reactions given below. On the basis of these reactions find out which of the algebraic relationship given in options (a) to (d) is correct?
(a)
(b)
(c)
(d)
Show Answer
Answer
(c) Same bonds are formed in reaction (1) and (2) but no bonds are broken in reaction (1) whereas bonds in the reactant molecules are broken in reaction (2). As energy is absorbed when bonds are broken, energy released in reaction (1) is greater than that in reaction (2) hence,
-
Option (a)
-
Option (b)
-
Option (d)
12. The enthalpies of elements in their standard states are taken as zero. The enthalpy of formation of a compound
(a) is always negative
(b) is always positive
(c) may be positive or negative
(d) is never negative
Show Answer
Answer
(c) Combustion of elements to form a compound can be exothermic or endothermic. e.g.,
whereas,
Hence, enthalpy of formation can be positive or negative.
-
(a) is always negative: This is incorrect because the enthalpy of formation can be either exothermic (negative) or endothermic (positive). Not all formation reactions release energy; some absorb energy.
-
(b) is always positive: This is incorrect because the enthalpy of formation can be either positive or negative. While some formation reactions absorb energy, others release energy.
-
(d) is never negative: This is incorrect because the enthalpy of formation can indeed be negative. Many formation reactions are exothermic, meaning they release energy and have a negative enthalpy change.
13. Enthalpy of sublimation of a substance is equal to
(a) enthalpy of fusion + enthalpy of vaporisation
(b) enthalpy of fusion
(c) enthalpy of vaporisation
(d) twice the enthalpy of vaporisation
Show Answer
Answer
(a) Enthalpy of sublimation of a substance is equal to enthalpy of fusion + enthalpy of vaporisation.
Sublimation is, direct conversion of solid to vapour. solid
Writing in two steps, we have solid
solid
liquid
-
(b) Enthalpy of fusion: This option is incorrect because the enthalpy of sublimation involves both the phase change from solid to liquid (enthalpy of fusion) and the phase change from liquid to vapor (enthalpy of vaporization). Enthalpy of fusion alone only accounts for the energy required to convert a solid to a liquid, not to a vapor.
-
(c) Enthalpy of vaporisation: This option is incorrect because the enthalpy of sublimation includes the energy required for both the solid to liquid transition (enthalpy of fusion) and the liquid to vapor transition (enthalpy of vaporization). Enthalpy of vaporization alone only accounts for the energy required to convert a liquid to a vapor, not from a solid to a vapor.
-
(d) Twice the enthalpy of vaporisation: This option is incorrect because the enthalpy of sublimation is not simply twice the enthalpy of vaporization. It is the sum of the enthalpy of fusion and the enthalpy of vaporization, which are distinct and separate energy changes.
14. Which of the following is not correct?
(a)
(b)
(c)
(d)
Show Answer
Answer
(b)
(i) If
(ii) If
(iii) If
-
Option (a) is correct because
-
Option (c) is correct because
-
Option (d) is correct because
Multiple Choice Questions (More Than One Options)
15. Thermodynamics mainly deals with
(a) interrelation of various forms of energy and their transformation from one form to another.
(b) energy changes in the processes which depend only on initial and final states of the microscopic systems containing a few molecules.
(c) how and at what rate these energy transformations are carried out.
(d) the system in equilibrium state or moving from one equilibrium state to another equilibrium state.
Show Answer
Answer
Thermodynamics deals with interrelation of various forms of energy and their transformation into each other. It also deals with thermal or mechanical equilibrium. However, it does not tell anything about the rate of reaction.
- Option (b) is incorrect because thermodynamics does not focus on processes involving only a few molecules; it generally deals with macroscopic systems containing a large number of molecules.
- Option (c) is incorrect because thermodynamics does not concern itself with the rate at which energy transformations occur; that is the domain of kinetics.
16. In an exothermic reaction, heat is evolved, and system loses heat to the surrounding. For such system
(a)
(b)
(c)
(d)
Show Answer
Answer
Exothermic reactions are those reactions which are accompanied by the evolution of heat.
e.g.,
-
(c)
-
(d)
17. The spontaneity means, having the potential to proceed without the assistance of external agency. The processes which occur spontaneously are
(a) flow of heat from colder to warmer body
(b) gas in a container contracting into one corner
(c) gas expanding to fill the available volume
(d) burning carbon in oxygen to give carbon dioxide
Show Answer
Answer
(c,
Options (a) and (b) can neither occur by themselves nor by initiation, (c) can occur by itself,
(d) occur on initiation. Flowing of heat from warmer to colder body, expanding of gas and burning of carbon to give carbon dioxide, all are spontaneous process.
-
Option (a) is incorrect because heat naturally flows from a warmer body to a colder body, not the other way around. The flow of heat from a colder to a warmer body would require external work or intervention, making it non-spontaneous.
-
Option (b) is incorrect because a gas naturally tends to expand and fill the available volume due to the increase in entropy. A gas contracting into one corner of a container would require external work or intervention, making it non-spontaneous.
18. For an ideal gas, the work of reversible expansion under isothermal condition can be calculated by using the expression
A sample containing
(a) Work done at
(b) Work done at
(c) Work done at
(d)
Show Answer
Answer
Given that, the work of reversible expansion under isothernal condition can be calculated by using the expression
Putting these values in above expression
For isothermal expansion of ideal gases,
-
Option (a): Work done at
-
Option (b): Work done at
19. Consider the following reaction between zinc and oxygen and choose the correct options out of the options given below
(a) The enthalpy of two moles of
(b) The enthalpy of two moles of
(c)
(d)
Show Answer
Answer
For the reaction,
As we know that,
A negative value of
-
Option (b) is incorrect because the enthalpy of two moles of ZnO is not more than the total enthalpy of two moles of Zn and one mole of oxygen by 693.8 kJ. The negative value of ΔH indicates that the enthalpy of the products (ZnO) is less than the enthalpy of the reactants (Zn and O₂).
-
Option (d) is incorrect because 693.8 kJ/mol of energy is not absorbed in the reaction. The negative ΔH value indicates that energy is released (evolved) during the reaction, not absorbed.
Short Answer Type Questions
20.
Show Answer
Answer
Given that, quantity of water
As we know that,
Enthalpy change for vaporising 1 mole of
Standard enthalpy of vaporisation at
Show Answer
Answer
One mole of acetone requires less heat to vaporise than 1 mole of water. Hence, acetone has less enthalpy of vaporisation and water has higher enthalpy of vaporisation. It can be represented as
Show Answer
Answer
No, the
This reaction is different from the given reaction.
Hence,
Show Answer
Answer
Given,
And for the reverse reaction,
Hence, the value of
Show Answer
Answer
In general, if enthalpy of an overall reaction
Note For a general reaction Hess’s law of constant heat summation can be represented as

Show Answer
Thinking Process
To solve this problem, keep in mind that in methane all the four
Answer
In
Show Answer
Thinking Process
This question is based upon the concept of Born-Haber cycle as well as Hess’s law. Following steps are used to solve this problem.
(i)
(ii)
(iii)
(iv)
(v) Applying Hess’s law
Answer
Given that,
IE of
Born-Haber cycle for the formation of

By applying Hess’s law,
Show Answer
Answer
The mixing of two gases have
Show Answer
Answer
Heat has randomising influence on a system and temperature is the measure of average chaotic motion of particles in the system. The mathematical relation which relates these three parameters is
Here,
Show Answer
Answer
Yes, the temperature of system and surroundings be the same when they are in thermal equilibrium.
Note Thermal equilibrium is defined as when two physical systems are brought into a connection that does not allow transfer of matter between them, and does not allow transfer of energy between them, such a connection is said to permit transfer of energy as heat, and is called diathermal.
If a diathermal connection is made between two physical systems and the making of the connection is followed by no change of state of either, then the two systems are said to be in relation of thermal equilibrium. It obeys zeroth law of thermodynamics.
Show Answer
Answer
For the reaction,
As we know that
Here,
Show Answer
Answer
The net enthalpy change,
Show Answer
Answer
The standard molar entropy of
Thus, molar entropy of
Show Answer
Answer
State functions are those values which depend only on the state of the system and not on how it is reached e.g., enthalpy, entropy, temperature and free energy. Path functions are those values which depend on the path of the system. e.
Show Answer
Answer
Amount of heat required to vaporise one mole of a liquid at constant temperature and under standard pressure ( 1 bar ) is called its molar enthalpy of vaporisation
Show Answer
Answer
Gibbs energy for a reaction in which all reactants and products are in standard state.
For all other values of
Show Answer
Answer
For isolated system there is no transfer of energy as heat, i.e.,
Show Answer
Answer
The two conditions under which heat becomes independent of path are
(i) when volume remains constant
(ii) when pressure remains constant
Explanation
(i) At constant volume By first law of thermodynamics,
Hence,
(ii) At constant pressure As we know,
Show Answer
Answer
Work done of a gas in vacuum,
i.e.,
It is to be remember that as
Show Answer
Answer
For water, molar heat capacity,
Show Answer
Answer
Given that,
Show Answer
Answer
Given that, enthalpy of combustion of
Molar enthalpy change for the combustion of graphite,
graphite
Negative sign in the value of
Show Answer
Thinking Process
To, calculate the enthalpy change, use the following formula when the reactants, and products are in gas phase as
Answer
Given that, bond energy of
bond energy of
bond energy of
For the reaction
Show Answer
Answer
Given that,
What will be standard enthalpy of formation of
Show Answer
Answer
Given that,
Enthalpy of formation is the enthalpy change of the reaction when 1 mole of the compound is formed from its elements then
This can be obtained by dividing the given equation by 2 .
Therefore,
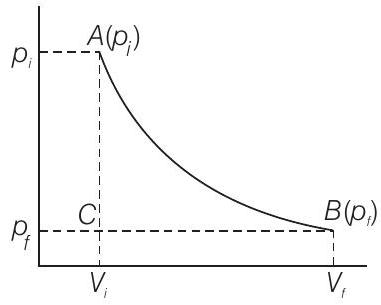
Show Answer
Answer
Suppose total volume of the gas is
Then volume change,
If
This can be calculated from

The negative sign in this expression is required to obtain conventional sign for
Show Answer
Answer
When compression is carried out in infinite steps with change in pressure, it is a reversible process. The work done can be calculated from

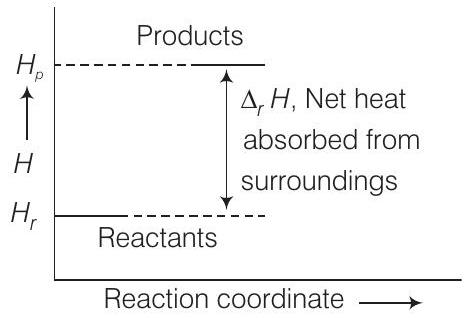
(a) Throwing a stone from the ground to roof.
(b)
In which of the processes potential energy/enthalpy change is contributing factor to the spontaneity?
Show Answer
Answer
Representation of potential energy/enthalpy change in the following processes
(a) Throwing a stone from the ground to roof.

(b)

Energy increases in (a) and it decreases in (b) process. Hence, in process (b), enthalpy change is the contributing factor to the spontaneity.
Show Answer
Answer
No, enthalpy is one of the contributing factors in deciding spontaneity but it is not the only factor. Another contributory factor, entropy factor has also to be taken into consideration.
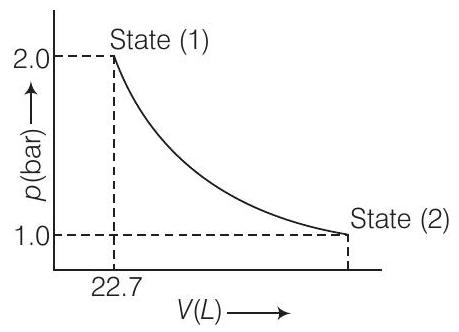
Show Answer
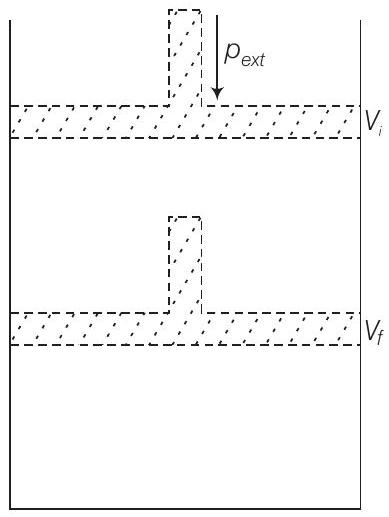
Answer
The given diagram represent that the process is carried out in infinite steps, hence it is isothermal reversible expansion of the ideal gas from pressure
(Given that,
Show Answer
Answer
In the first case, as the expansion is against constant external pressure
$$ \begin{aligned} W & =-{\text {ext }}\left(V{2}-V_{1}\right)=-2 \operatorname{bar} \times(50-10) \mathrm{L} \ & =-80 \mathrm{~L} \text { bar } \quad \quad (1L bar = 100 J)\ & =-80 \times 100 \mathrm{~J} \ & =-8 \mathrm{~kJ} \end{aligned} $$
If the given expansion was carried out reversibly, the internal pressure of the gas should be greater than the external pressure at every stage. Hence, the work done will be more.
Matching The Columns
51. Match the following.
| List I | List II | ||
|---|---|---|---|
| A. | Adiabatic process | 1. | Heat |
| B. | Isolated system | 2. | At constant volume |
| C. | Isothermal change | 3. | First law of thermodynamics |
| D. | Path function | 4. | No exchange of energy and matter |
| E. | State function | 5. | No transfer of heat |
| F. | 6. | Constant temperature | |
| G. | Law of conservation of energy | 7. | Internal energy |
| H. | Reversible process | 8. | Pext = 0 |
| I. | Free expansion | 9. | At constant pressure |
| J. | 10. | Infinitely slow process which | |
| proceeds through a series of | |||
| K. | Intensive property | 11. | Entropy |
| L. | Extensive property | 12. | Pressure |
| 13. | Specific heat |
Show Answer
Answer
A.
B.
C.
D.
E.
F.
G.
H.
I.
J.
K.
L.
Correct Matching can be done as
| A. | Adiabatic process | No transfer of heat |
|---|---|---|
| B. | Isolated system | No exchange of energy and matter |
| C. | Isothermal change | Constant temperature |
| D. | Path function | Heat |
| E. | State function | Internal energy |
| Entropy | ||
| Pressure | ||
| F. | At constant volume | |
| G. | Law of conservation of energy | First law of thermodynamics |
| H. | Reversible process | Infinitely slow process which |
| proceeds through a series of | ||
| I. | Free expansion | equilibrium states |
| J. | Pext |
|
| K. | Intensive property | At constant pressure |
| Heat | ||
| Pressure | ||
| L. | Extensive property | Specific heat |
| Reaction | Entropy change | ||
|---|---|---|---|
| A. | A liquid vaporises | 1. | |
| B. | Reaction is non-spontaneous at all temperatures and |
2. | |
| C. | Reversible expansion of an ideal gas | 3. |
Show Answer
Answer
A.
B.
C.
A. When a liquid vaporises, i.e., liquid
B. When
C. In the reversible expansion of an ideal gas, the system remains in equilibrium at every stage. Hence,
Certainly! Here’s the table with separate columns for each parameter:
| Description | ||||
|---|---|---|---|---|
| A. | (+) | (+) | (+) | 1. Non-spontaneous at high temperature |
| B. | (-) | (-) | (+) at high (T) | 2. Spontaneous at all temperatures |
| C. | (-) | (+) | (-) | 3. Non-spontaneous at all temperatures |
Show Answer
Answer
A.
B.
C.
A. When
B. When
C. When
| A. | Entropy of vaporisation | 1. | decreases |
|---|---|---|---|
| B. | 2. | is always positive | |
| C. | Crystalline solid state | 3. | lowest entropy |
| D. | 4. |
Show Answer
Answer
A.
B.
C.
D.
A. Entropy of vaporisation is always positive. It is equal to
B.
If
C. Crystalline solid state has lowest entropy.
D. During adiabatic expansion of an ideal gas,
In the following questions a statement of assertion (A) followed by a statement of reason
Assertion (A) Combustion of all organic compounds is an exothermic reaction.
Reason (R) The enthalpies of all elements in their standard state are zero.
(a) Both
(b) Both
(c)
(d)
Show Answer
Answer
(b) Both assertion and reason are true but reason is not the correct explanation of assertion.
Correct explanation In a combustion reaction, sum of enthalpies of reactants is greater than the sum of the enthalpies of products.
Reason (R) Decrease in enthalpy is a contributory factor for spontaneity.
(a) Both
(b) Both
(c)
(d)
Show Answer
Answer
(b) Both assertion and reason are true but reason is not the correct explanation of assertion.
Spontaneous processes are accompanied by decrease in energy and increase in randomness.
Reason (R) In crystals, molecules organise in an ordered manner.
(a) Both
(b) Both
(c)
(d)
Show Answer
Answer
(a) Both assertion and reason are true and reason is the correct explanation of assertion. When a liquid crystallises, entropy decreases because in crystalline form the molecules are more ordered as compared to the liquid.
Long Answer Type Questions
58. Derive the relationship between
Show Answer
Answer
From the first law of thermodynamics,
If the process carried out at constant volume,
Hence,
Similarly,
Here,
Enthalpy change of a system is equal to the heat absorbed or evolved by the system at constant pressure.
As we know that at constant pressure,
where,
This equation can be rewritten as
where,
But for the ideal gases,
So that
and
where,
Substituting these values in Eq. (i), we get
where,
Putting the values of
Note Conditions under which
(i) When reaction is carried out in a closed vessel so that volume remains constant i.e.,
(ii) When reaction involves only solids or liquids or solutions but no gaseous reactant or product. This is because the volume changes of the solids and liquids during a chemical reaction are negligible.
(iii) When reaction involves gaseous reactants and products but their number of moles are equal (i.e.,
Since,
Mass, internal energy, pressure, heat capacity, molar heat capacity, density, mole fraction, specific heat, temperature and molarity.
Show Answer
Answer
Extensive properties Those properties whose value depends on the quantity or size of matter present in the system is known as extensive properties.
e.g., mass, internal energy, heat capacity.
Intensive properties Those properties which do not depend on the quantity or size of matter present are known as intensive properties. e.g., pressure, molar heat capacity, density, mole fraction, specific heat, temperature and molarity.
Mole fraction or molarity of a solution is same whether we take a small amount of the solution or large amount of the solution.
Ratio of two extensive properties is always intensive.
So, mole fraction and molarity are intensive properties.
Show Answer
Answer
The lattice enthalpy of an ionic compound is the enthalpy change which occurs when one mole of an ionic compound dissociates into its ions in gaseous state. For the reaction
Since, it is impossible to determine lattice enthalpies directly by experiment, we use an indirect method where we construct an enthalpy diagram called a Born-Haber cycle.
Let us now calculate the lattice enthalpy of
(i)
(ii)
(iii)
(iv)

Enthalply diagram for lattice enthalpy of
(v)
The sequence of steps is shown in given figure and is known as Born-Haber cycle. The importance of the cycle is that, the sum of the enthalpy changes round a cycle is zero.
Applying Hess’s law, we get
Show Answer
Answer
Gibbs free energy is that thermodynamic quantity of a system, the decrease in whose value during a process is equal to the maximum possible useful work that can be obtained from the system.
Mathematically, this results may be derived as follows
The relationship between heat absorbed by a system
Under constant pressure condition, the expansion work is given by
For a reversible change taking place at constant temperature,
Substituting the value of
For a change taking place under conditions of constant temperature and pressure,
Substituting this value in equation (iv), we get
Thus, free energy change can be taken as a measure of work other than the work of expansion. For most changes, the work of expansion can not be converted to other useful work, whereas the non-expansion work is convertible to useful work.
Rearranging equation
As
If
Show Answer
Answer
(i) Total work done in an expansion when the state of an ideal gas is changed reversibly and isothermally from
(ii) Work against constant pressure,