Some Basic Concepts of Chemistry
“Chemistry is the science of molecules and their transformations. It is the science not so much of the one hundred elements but of the infinite variety of molecules that may be built from them.”
Roald Hoffmann
Science can be viewed as a continuing human effort to systematise knowledge for describing and understanding nature. You have learnt in your previous classes that we come across diverse substances present in nature and changes in them in daily life. Curd formation from milk, formation of vinegar from sugarcane juice on keeping for prolonged time and rusting of iron are some of the examples of changes which we come across many times. For the sake of convenience, science is sub-divided into various disciplines: chemistry, physics, biology, geology, etc. The branch of science that studies the preparation, properties, structure and reactions of material substances is called chemistry.
DEVELOPMENT OF CHEMISTRY
Chemistry, as we understand it today, is not a very old discipline. Chemistry was not studied for its own sake, rather it came up as a result of search for two interesting things:
i. Philosopher’s stone (Paras) which would convert all baser metals e.g., iron and copper into gold.
ii. ‘Elexir of life’ which would grant immortality.
People in ancient India, already had the knowledge of many scientific phenomenon much before the advent of modern science. They applied that knowledge in various walks of life. Chemistry developed mainly in the form of Alchemy and Iatrochemistry during 1300-1600 CE. Modern chemistry took shape in the
Other cultures - especially the Chinese and the Indian - had their own alchemical traditions. These included much knowledge of chemical processes and techniques.
In ancient India, chemistry was called Rasayan Shastra, Rastantra, Ras Kriya or Rasvidya. It included metallurgy, medicine, manufacture of cosmetics, glass, dyes, etc. Systematic excavations at Mohenjodaro in Sindh and Harappa in Punjab prove that the story of development of chemistry in India is very old. Archaeological findings show that baked bricks were used in construction work. It shows the mass production of pottery, which can be regarded as the earliest chemical process, in which materials were mixed, moulded and subjected to heat by using fire to achieve desirable qualities. Remains of glazed pottery have been found in Mohenjodaro. Gypsum cement has been used in the construction work. It contains lime, sand and traces of
Copper metallurgy in India dates back to the beginning of chalcolithic cultures in the subcontinent. There are much archeological evidences to support the view that technologies for extraction of copper and iron were developed indigenously.
According to Rigveda, tanning of leather and dying of cotton were practised during
A vast number of statements and material described in the ancient Vedic literature can be shown to agree with modern scientific findings. Copper utensils, iron, gold, silver ornaments and terracotta discs and painted grey pottery have been found in many archaeological sites in north India. Sushruta Samhita explains the importance of Alkalies. The Charaka Samhita mentions ancient indians who knew how to prepare sulphuric acid, nitric acid and oxides of copper, tin and zinc; the sulphates of copper, zinc and iron and the carbonates of lead and iron.
Rasopanishada describes the preparation of gunpowder mixture. Tamil texts also describe the preparation of fireworks using sulphur, charcoal, saltpetre (i.e., potassium nitrate), mercury, camphor, etc.
Nagarjuna was a great Indian scientist. He was a reputed chemist, an alchemist and a metallurgist. His work Rasratnakar deals with the formulation of mercury compounds. He has also discussed methods for the extraction of metals, like gold, silver, tin and copper. A book, Rsarnavam, appeared around
Chakrapani discovered mercury sulphide. The credit for inventing soap also goes to him. He used mustard oil and some alkalies as ingredients for making soap. Indians began making soaps in the
The paintings found on the walls of Ajanta and Ellora, which look fresh even after ages, testify to a high level of science achieved in ancient India. Varähmihir’s Brihat Samhita is a sort of encyclopaedia, which was composed in the sixth century
A number of classical texts, like Atharvaveda (1000 BCE) mention some dye stuff, the material used were turmeric, madder, sunflower, orpiment, cochineal and lac. Some other substances having tinting property were kamplcica, pattanga and jatuka.
Varähmihir’s Brihat Samhita gives references to perfumes and cosmetics. Recipes for hair dying were made from plants, like indigo and minerals like iron power, black iron or steel and acidic extracts of sour rice gruel. Gandhayukli describes recipes for making scents, mouth perfumes, bath powders, incense and talcum power.
Paper was known to India in the
It seems that the process of fermentation was well-known to Indians. Vedas and Kautilya’s Arthashastra mention about many types of liquors. Charaka Samhita also mentions ingredients, such as barks of plants, stem, flowers, leaves, woods, cereals, fruits and sugarcane for making Asavas.
The concept that matter is ultimately made of indivisible building blocks, appeared in India a few centuries BCE as a part of philosophical speculations. Acharya Kanda, born in
Charaka Samhita is the oldest Ayurvedic epic of India. It describes the treatment of diseases. The concept of reduction of particle size of metals is clearly discussed in Charaka Samhita. Extreme reduction of particle size is termed as nanotechnology. Charaka Samhita describes the use of bhasma of metals in the treatment of ailments. Now-a-days, it has been proved that bhasmas have nanoparticles of metals.
After the decline of alchemy, Iatrochemistry reached a steady state, but it too declined due to the introduction and practise of western medicinal system in the
From the above discussion, you have learnt that chemistry deals with the composition, structure, properties and interection of matter and is of much use to human beings in daily life. These aspects can be best described and understood in terms of basic constituents of matter that are atoms and molecules. That is why, chemistry is also called the science of atoms and molecules. Can we see, weigh and perceive these entities (atoms and molecules)? Is it possible to count the number of atoms and molecules in a given mass of matter and have a quantitative relationship between the mass and the number of these particles? We will get the answer of some of these questions in this Unit. We will further describe how physical properties of matter can be quantitatively described using numerical values with suitable units.
1.1 IMPORTANCE OF CHEMISTRY
Chemistry plays a central role in science and is often intertwined with other branches of science.
Principles of chemistry are applicable in diverse areas, such as weather patterns, functioning of brain and operation of a computer, production in chemical industries, manufacturing fertilisers, alkalis, acids, salts, dyes, polymers, drugs, soaps, detergents, metals, alloys, etc., including new material.
Chemistry contributes in a big way to the national economy. It also plays an important role in meeting human needs for food, healthcare products and other material aimed at improving the quality of life. This is exemplified by the large-scale production of a variety of fertilisers, improved variety of pesticides and insecticides. Chemistry provides methods for the isolation of lifesaving drugs from natural sources and makes possible synthesis of such drugs. Some of these drugs are cisplatin and taxol, which are effective in cancer therapy. The drug AZT (Azidothymidine) is used for helping AIDS patients.
Chemistry contributes to a large extent in the development and growth of a nation. With a better understanding of chemical principles it has now become possible to design and synthesise new material having specific magnetic, electric and optical properties. This has lead to the production of superconducting ceramics, conducting polymers, optical fibres, etc. Chemistry has helped in establishing industries which manufacture utility goods, like acids, alkalies, dyes, polymesr metals, etc. These industries contribute in a big way to the economy of a nation and generate employment.
In recent years, chemistry has helped in dealing with some of the pressing aspects of environmental degradation with a fair degree of success. Safer alternatives to environmentally hazardous refrigerants, like CFCs (chlorofluorocarbons), responsible for ozone depletion in the stratosphere, have been successfully synthesised. However, many big environmental problems continue to be matters of grave concern to the chemists. One such problem is the management of the Green House gases, like methane, carbon dioxide, etc. Understanding of biochemical processes, use of enzymes for large-scale production of chemicals and synthesis of new exotic material are some of the intellectual challenges for the future generation of chemists. A developing country, like India, needs talented and creative chemists for accepting such challenges. To be a good chemist and to accept such challanges, one needs to understand the basic concepts of chemistry, which begin with the concept of matter. Let us start with the nature of matter.
1.2 NATURE OF MATTER
You are already familiar with the term matter from your earlier classes. Anything which has mass and occupies space is called matter. Everything around us, for example, book, pen, pencil, water, air, all living beings, etc., are composed of matter. You know that they have mass and they occupy space. Let us recall the characteristics of the states of matter, which you learnt in your previous classes.
1.2.1 States of Matter
You are aware that matter can exist in three physical states viz. solid, liquid and gas. The constituent particles of matter in these three states can be represented as shown in Fig. 1.1.
Particles are held very close to each other in solids in an orderly fashion and there is not much freedom of movement. In liquids, the particles are close to each other but they can move around. However, in gases, the particles are far apart as compared to those present in solid or liquid states and their movement is easy and fast. Because of such arrangement of particles, different states of matter exhibit the following characteristics:
(i) Solids have definite volume and definite shape.
(ii) Liquids have definite volume but do not have definite shape. They take the shape of the container in which they are placed.

(iii) Gases have neither definite volume nor definite shape. They completely occupy the space in the container in which they are placed.
These three states of matter are interconvertible by changing the conditions of temperature and pressure.
Solid
On heating, a solid usually changes to a liquid, and the liquid on further heating changes to gas (or vapour). In the reverse process, a gas on cooling liquifies to the liquid and the liquid on further cooling freezes to the solid.
1.2.2. Classification of Matter
In Class IX (Chapter 2), you have learnt that at the macroscopic or bulk level, matter can be classified as mixture or pure substance. These can be further sub-divided as shown in Fig. 1.2.
When all constituent particles of a substance are same in chemical nature, it is said to be a pure substance. A mixture contains many types of particles.
A mixture contains particles of two or more pure substances which may be present in it in any ratio. Hence, their composition is variable. Pure substances forming mixture are called its components. Many of the substances present around you are mixtures. For example, sugar solution in water, air, tea, etc., are all mixtures. A mixture may be homogeneous or heterogeneous. In a homogeneous mixture, the components completely mix with each other. This means particles of components of the mixture are uniformly distributed throughout the bulk of the mixture and its composition is uniform throughout. Sugar solution and air are the examples of homogeneous mixtures. In contrast to this, in a heterogeneous mixture, the composition is not uniform throughout and sometimes different components are visible. For example, mixtures of salt and sugar, grains and pulses along with some dirt (often stone pieces), are heterogeneous mixtures. You can think of many more examples of mixtures which you come across in the daily life. It is worthwhile to mention here that the components of a mixture can be separated by using physical methods, such as simple hand-picking, filtration, crystallisation, distillation, etc.

Pure substances have characteristics different from mixtures. Constituent particles of pure substances have fixed composition. Copper, silver, gold, water and glucose are some examples of pure substances. Glucose contains carbon, hydrogen and oxygen in a fixed ratio and its particles are of same composition. Hence, like all other pure substances, glucose has a fixed composition. Also, its constituents-carbon, hydrogen and oxygen - cannot be separated by simple physical methods.
Pure substances can further be classified into elements and compounds. Particles of an element consist of only one type of atoms. These particles may exist as atoms or molecules. You may be familiar with atoms and molecules from the previous classes; however, you will be studying about them in detail in Unit 2. Sodium, copper, silver, hydrogen, oxygen, etc., are some examples of elements. Their all atoms are of one type. However, the atoms of different elements are different in nature. Some elements, such as sodium or copper, contain atoms as their constituent particles, whereas, in some others, the constituent particles are molecules which are formed by two or more atoms. For example, hydrogen, nitrogen and oxygen gases consist of molecules, in which two atoms combine to give their respective molecules. This is illustrated in Fig. 1.3.

When two or more atoms of different elements combine together in a definite ratio, the molecule of a compound is obtained. Moreover, the constituents of a compound cannot be separated into simpler substances by physical methods. They can be separated by chemical methods. Examples of some compounds are water, ammonia, carbon dioxide, sugar, etc. The molecules of water and carbon dioxide are represented in Fig. 1.4.

Note that a water molecule comprises two hydrogen atoms and one oxygen atom. Similarly, a molecule of carbon dioxide contains two oxygen atoms combined with one carbon atom. Thus, the atoms of different elements are present in a compound in a fixed and definite ratio and this ratio is characteristic of a particular compound. Also, the properties of a compound are different from those of its constituent elements. For example, hydrogen and oxygen are gases, whereas, the compound formed by their combination i.e., water is a liquid. It is interesting to note that hydrogen burns with a pop sound and oxygen is a supporter of combustion, but water is used as a fire extinguisher.
1.3 PROPERTIES OF MATTER AND THEIR MEASUREMENT
1.3.1 Physical and chemical properties
Every substance has unique or characteristic properties. These properties can be classified into two categories - physical properties, such as colour, odour, melting point, boiling point, density, etc., and chemical properties, like composition, combustibility, ractivity with acids and bases, etc.
Physical properties can be measured or observed without changing the identity or the composition of the substance. The measurement or observation of chemical properties requires a chemical change to occur. Measurement of physical properties does not require occurance of a chemical change. The examples of chemical properties are characteristic reactions of different substances; these include acidity or basicity, combustibility, etc. Chemists describe, interpret and predict the behaviour of substances on the basis of knowledge of their physical and chemical properties, which are determined by careful measurement and experimentation. In the following section, we will learn about the measurement of physical properties.
1.3.2 Measurement of physical properties
Quantitative measurement of properties is reaquired for scientific investigation. Many properties of matter, such as length, area, volume, etc., are quantitative in nature. Any quantitative observation or measurement is represented by a number followed by units in which it is measured. For example, length of a room can be represented as
Earlier, two different systems of measurement, i.e., the English System and the Metric System were being used in different parts of the world. The metric system, which originated in France in late eighteenth century, was more convenient as it was based on the decimal system. Late, need of a common standard system was felt by the scientific community. Such a system was established in 1960 and is discussed in detail below.
1.3.3 The International System of Units (SI)
The International System of Units (in French Le Systeme International d’Unités - abbreviated as SI) was established by the
The SI system has seven base units and they are listed in Table 1.1. These units pertain to the seven fundamental scientific quantities. The other physical quantities, such as speed, volume, density, etc., can be derived from these quantities.
Table 1.1 Base Physical Quantities and their Units
| Base Physical Quantity |
Symbol for Quantity |
Name of SI Unit |
Symbol for SI Unit |
|---|---|---|---|
| Length | metre | ||
| Mass | kilogram | ||
| Time | second | ||
| Electric current | ampere | ||
| Thermodynamic temperature | kelvin | ||
| Amount of substance | mole | ||
| Luminous intensity | candela |
The definitions of the SI base units are given in Table 1.2.
The SI system allows the use of prefixes to indicate the multiples or submultiples of a unit. These prefixes are listed in Table 1.3.
Let us now quickly go through some of the quantities which you will be often using in this book.
Table 1.2 Definitions of SI Base Units
| Unit of length | metre | The metre, symbol |
| Unit of mass | kilogram | The kilogram, symbol kg. is the SI unit of mass. It is defined by taking the fixed numerical value of the Planck constant |
| Unit of time | second | The second symbol |
| Unit of electric current | amphere | The ampere, symbol A, is the SI unit of electric current. It is defined by taking the fixed numerical value of the elementary charge |
| Unit of thermodynamic temperature | kelvin | The Kelvin, symbol |
| Unit of amount of substance | mole | The mole, symbol mol, is the SI unit of amount of substance. One mole contains exactly |
| Unit of luminous Intensity | Candela | The candela, symbol cd is the SI unit of luminous intensity in a given direction. It is defined by taking the fixed numerical value of the luminous efficacy of monochromatic radiation of frequency |
Table 1.3 Prefixes used in the SI System
| Multiple | Prefix | Symbol |
|---|---|---|
| yocto | ||
| zepto | ||
| atto | ||
| femto | ||
| pico | ||
| nano | ||
| micro | ||
| milli | ||
| centi | ||
| deci | ||
| 10 | deca | |
| hecto | ||
| kilo | ||
| mega | ||
| giga | ||
| tera | ||
| peta | ||
| exa | ||
| zeta | ||
| yotta |
Maintaining the National Standards of Measurement
The system of units, including unit definitions, keeps on changing with time. Whenever the accuracy of measurement of a particular unit was enhanced substantially by adopting new principles, member nations of metre treaty (signed in 1875), agreed to change the formal definition of that unit. Each modern industrialised country, including India, has a National Metrology Institute (NMI), which maintains standards of measurements. This responsibility has been given to the National Physical Laboratory (NPL), New Delhi. This laboratory establishes experiments to realise the base units and derived units of measurement and maintains National Standards of Measurement. These standards are periodically inter-compared with standards maintained at other National Metrology Institutes in the world, as well as those, established at the International Bureau of Standards in Paris.
1.3.4 Mass and Weight
Mass of a substance is the amount of matter present in it, while weight is the force exerted by gravity on an object. The mass of a substance is constant, whereas, its weight may vary from one place to another due to change in gravity. You should be careful in using these terms.
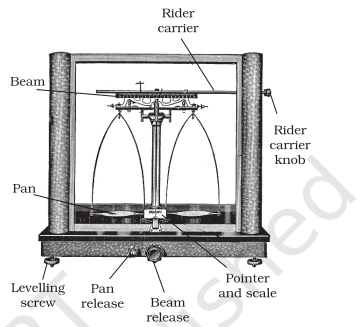
The mass of a substance can be determined accurately in the laboratory by using an analytical balance (Fig. 1.5).
The SI unit of mass as given in Table 1.1 is kilogram. However, its fraction named as gram
1.3.5 Volume
Volume is the amont of space occupied by a substance. It has the units of (length)
A common unit, litre (L) which is not an SI unit, is used for measurement of volume of liquids.
Fig. 1.6 helps to visualise these relations.

In the laboratory, the volume of liquids or solutions can be measured by graduated cylinder, burette, pipette, etc. A volumetric flask is used to prepare a known volume of a solution. These measuring devices are shown in Fig. 1.7.

1.3.6 Density
The two properties - mass and volume discussed above are related as follows:
Density of a substance is its amount of mass per unit volume. So, SI units of density can be obtained as follows:
This unit is quite large and a chemist often expresses density in
1.3.7 Temperature
There are three common scales to measure temperature
The temperatures on two scales are related to each other by the following relationship:
The kelvin scale is related to celsius scale as follows:
It is interesting to note that temperature below

1.4 UNCERTAINTY IN MEASUREMENT
Many a time in the study of chemistry, one has to deal with experimental data as well as theoretical calculations. There are meaningful ways to handle the numbers conveniently and present the data realistically with certainty to the extent possible. These ideas are discussed below in detail.
Reference Standard
After defining a unit of measurement such as the kilogram or the metre, scientists agreed on reference standards that make it possible to calibrate all measuring devices. For getting reliable measurements, all devices such as metre sticks and analytical balances have been calibrated by their manufacturers to give correct readings. However, each of these devices is standardised or calibrated against some reference. The mass standard is the kilogram since 1889. It has been defined as the mass of platinum-iridium (Pt-Ir) cylinder that is stored in an airtight jar at International Bureau of Weights and Measures in Sevres, France. Pt-Ir was chosen for this standard because it is highly resistant to chemical attack and its mass will not change for an extremely long time.
Scientists are in search of a new standard for mass. This is being attempted through accurate determination of Avogadro constant. Work on this new standard focuses on ways to measure accurately the number of atoms in a well-defined mass of sample. One such method, which uses X-rays to determine the atomic density of a crystal of ultrapure silicon, has an accuracy of about 1 part in
The metre was originally defined as the length between two marks on a Pt-Ir bar kept at a temperature of
1.4.1 Scientific Notation
As chemistry is the study of atoms and molecules, which have extremely low masses and are present in extremely large numbers, a chemist has to deal with numbers as large as
It may look funny for a moment to write or count numbers involving so many zeros but it offers a real challenge to do simple mathematical operations of addition, subtraction, multiplication or division with such numbers. You can write any two numbers of the above type and try any one of the operations you like to accept as a challenge, and then, you will really appreciate the difficulty in handling such numbers.
This problem is solved by using scientific notation for such numbers, i.e., exponential notation in which any number can be represented in the form
Thus, we can write 232.508 as
Similarly, 0.00016 can be written as
While performing mathematical operations on numbers expressed in scientific notations, the following points are to be kept in mind.
Multiplication and Division
These two operations follow the same rules which are there for exponential numbers, i.e.
Addition and Subtraction
For these two operations, first the numbers are written in such a way that they have the same exponent. After that, the coefficients (digit terms) are added or subtracted as the case may be.
Thus, for adding
Then, these numbers can be added as follows
Similarly, the subtraction of two numbers can be done as shown below:
1.4.2 Significant Figures
Every experimental measurement has some amount of uncertainty associated with it because of limitation of measuring instrument and the skill of the person making the measurement. For example, mass of an object is obtained using a platform balance and it comes out to be
The uncertainty in the experimental or the calculated values is indicated by mentioning the number of significant figures. Significant figures are meaningful digits which are known with certainty plus one which is estimated or uncertain. The uncertainty is indicated by writing the certain digits and the last uncertain digit. Thus, if we write a result as
There are certain rules for determining the number of significant figures. These are stated below:
(1) All non-zero digits are significant. For example in
(2) Zeros preceding to first non-zero digit are not significant. Such zero indicates the position of decimal point. Thus, 0.03 has one significant figure and 0.0052 has two significant figures.
(3) Zeros between two non-zero digits are significant. Thus, 2.005 has four significant figures.
(4) Zeros at the end or right of a number are significant, provided they are on the right side of the decimal point. For example,
(5) Counting the numbers of object, for example, 2 balls or 20 eggs, have infinite significant figures as these are exact numbers and can be represented by writing infinite number of zeros after placing a decimal i.e.,
In numbers written in scientific notation, all digits are significant e.g.,
However, one would always like the results to be precise and accurate. Precision and accuracy are often referred to while we talk about the measurement.
Precision refers to the closeness of various measurements for the same quantity. However, accuracy is the agreement of a particular value to the true value of the result. For example, if the true value for a result is
Table 1.4 Data to Illustrate Precision and Accuracy
| Measurements/g | |||
|---|---|---|---|
| 1 | 2 | Average (g) | |
| Student A | 1.95 | 1.93 | 1.940 |
| Student B | 1.94 | 2.05 | 1.995 |
| Student C | 2.01 | 1.99 | 2.000 |
Addition and Subtraction of Significant Figures
The result cannot have more digits to the right of the decimal point than either of the original numbers.
Here, 18.0 has only one digit after the decimal point and the result should be reported only up to one digit after the decimal point, which is 31.1
Multiplication and Division of Significant Figures
In these operations, the result must be reported with no more significant figures as in the measurement with the few significant figures.
Since 2.5 has two significant figures, the result should not have more than two significant figures, thus, it is 3.1.
While limiting the result to the required number of significant figures as done in the above mathematical operation, one has to keep in mind the following points for rounding off the numbers
1. If the rightmost digit to be removed is more than 5, the preceding number is increased by one. For example, 1.386. If we have to remove 6 , we have to round it to 1.39 .
2. If the rightmost digit to be removed is less than 5 , the preceding number is not changed. For example, 4.334 if 4 is to be removed, then the result is rounded upto 4.33.
3. If the rightmost digit to be removed is 5 , then the preceding number is not changed if it is an even number but it is increased by one if it is an odd number. For example, if 6.35 is to be rounded by removing 5 , we have to increase 3 to 4 giving 6.4 as the result. However, if 6.25 is to be rounded off it is rounded off to 6.2.
1.4.3 Dimensional Analysis
Often while calculating, there is a need to convert units from one system to the other. The method used to accomplish this is called factor label method or unit factor method or dimensional analysis. This is illustrated below.
1.5 LAWS OF CHEMICAL COMBINATIONS
The combination of elements to form compounds is governed by the following five basic laws.

1.5.1 Law of Conservation of Mass
This law was put forth by Antoine Lavoisier in 1789. He performed careful experimental studies for combustion reactions and reached to the conclusion that in all physical and chemical changes, there is no net change in mass duting the process. Hence, he reached to the conclusion that matter can neither be created nor destroyed. This is called ‘Law of Conservation of Mass’. This law formed the basis for several later developments in chemistry. Infact, this was the result of exact measurement of masses of reactants and products, and carefully planned experiments performed by Lavoisier.
1.5.2 Law of Definite Proportions
This law was given by, a French chemist, Joseph Proust. He stated that a given compound always contains exactly the same proportion of elements by weight.
.png)
Proust worked with two samples of cupric carbonate - one of which was of natural origin and the other was synthetic. He found that the composition of elements present in it was same for both the samples as shown below:
| % of copper |
% of carbon |
% of oxygen |
|
|---|---|---|---|
| Natural Sample | 51.35 | 9.74 | 38.91 |
| Synthetic Sample | 51.35 | 9.74 | 38.91 |
Thus, he concluded that irrespective of the source, a given compound always contains same elements combined together in the same proportion by mass. The validity of this law has been confirmed by various experiments. It is sometimes also referred to as Law of Definite Composition.
1.5.3 Law of Multiple Proportions
This law was proposed by Dalton in 1803. According to this law, if two elements can combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element, are in the ratio of small whole numbers.
For example, hydrogen combines with oxygen to form two compounds, namely, water and hydrogen peroxide.
Here, the masses of oxygen (i.e.,
1.5.4 Gay Lussa’s Law of Gaseous Volumes
This law was given by Gay Lussac in 1808 . He observed that when gases combine or are produced in a chemical reaction they do so in a simple ratio by volume, provided all gases are at the same temperature and pressure.

Thus,
Thus, the volumes of hydrogen and oxygen which combine (i.e.,
Gay Lussac’s discovery of integer ratio in volume relationship is actually the law of definite proportions by volume. The law of definite proportions, stated earlier, was with respect to mass. The Gay Lussac’s law was explained properly by the work of Avogadro in 1811.
1.5.5 Avogadro’s Law
In 1811, Avogadro proposed that equal volumes of all gases at the same temperature and pressure should contain equal number of molecules. Avogadro made a distinction between atoms and molecules which is quite understandable in present times. If we consider again the reaction of hydrogen and oxygen to produce water, we see that two volumes of hydrogen combine with one volume of oxygen to give two volumes of water without leaving any unreacted oxygen.
.png)
Note that in the Fig. 1.9 (Page 16) each box contains equal number of molecules. In fact, Avogadro could explain the above result by considering the molecules to be polyatomic. If hydrogen and oxygen were considered as diatomic as recognised now, then the above results are easily understandable. However, Dalton and others believed at that time that atoms of the same kind cannot combine and molecules of oxygen or hydrogen containing two atoms did not exist. Avogadro’s proposal was published in the French Journal de Physique. In spite of being correct, it did not gain much support.

After about 50 years, in 1860, the first international conference on chemistry was held in Karlsruhe, Germany, to resolve various ideas. At the meeting, Stanislao Cannizaro presented a sketch of a course of chemical philosophy, which emphasised on the importance of Avogadro’s work.
1.6 DALTON’S ATOMIC THEORY
Although the origin of the idea that matter is composed of small indivisible particles called ‘a-tomio’ (meaning, indivisible), dates back to the time of Democritus, a Greek Philosopher (460
.png)
In 1808, Dalton published ‘A New System of Chemical Philosophy’, in which he proposed the following:
1. Matter consists of indivisible atoms.
2. All atoms of a given element have identical properties, including identical mass. Atoms of different elements differ in mass.
3. Compounds are formed when atoms of different elements combine in a fixed ratio.
4. Chemical reactions involve reorganisation of atoms. These are neither created nor destroyed in a chemical reaction.
Dalton’s theory could explain the laws of chemical combination. However, it could not explain the laws of gaseous volumes. It could not provide the reason for combining of atoms, which was answered later by other scientists.
1.7 ATOMIC AND MOLECULAR MASSES
After having some idea about the terms atoms and molecules, it is appropriate here to understand what do we mean by atomic and molecular masses.
1.7.1 Atomic Mass
The atomic mass or the mass of an atom is actually very-very small because atoms are extremely small. Today, we have sophisticated techniques e.g., mass spectrometry for determining the atomic masses fairly accurately. But in the nineteenth century, scientists could determine the mass of one atom relative to another by experimental means, as has been mentioned earlier. Hydrogen, being the lightest atom was arbitrarily assigned a mass of 1 (without any units) and other elements were assigned masses relative to it. However, the present system of atomic masses is based on carbon-12 as the standard and has been agreed upon in 1961. Here, Carbon-12 is one of the isotopes of carbon and can be represented as
And
Mass of an atom of hydrogen
Thus, in terms of amu, the mass
Similarly, the mass of oxygen -
At present, ‘amu’ has been replaced by ’
When we use atomic masses of elements in calculations, we actually use average atomic masses of elements, which are explained below.
1.7.2 Average Atomic Mass
Many naturally occurring elements exist as more than one isotope. When we take into account the existence of these isotopes and their relative abundance (per cent occurrence), the average atomic mass of that element can be computed. For example, carbon has the following three isotopes with relative abundances and masses as shown against each of them.
| Isotope | Relative Abundance (%) |
Atomic Mass (amu) |
|---|---|---|
| 98.892 | 12 | |
| 1.108 | 13.00335 | |
| 14.00317 |
From the above data, the average atomic mass of carbon will come out to be:
Similarly, average atomic masses for other elements can be calculated. In the periodic table of elements, the atomic masses mentioned for different elements actually represent their average atomic masses.
1.7.3 Molecular Mass
b Molecular mass is the sum of atomic masses of the elements present in a molecule. It is obtained by multiplying the atomic mass of each element by the number of its atoms and adding them together. For example, molecular mass of methane, which contains one carbon atom and four hydrogen atoms, can be obtained as follows:
Molecular mass of methane,
Similarly, molecular mass of water
1.7.4 Formula Mass
Some substances, such as sodium chloride, do not contain discrete molecules as their constituent units. In such compounds, positive (sodium ion) and negative (chloride ion) entities are arranged in a three-dimensional structure, as shown in Fig. 1.10.

It may be noted that in sodium chloride, one
The formula, such as
Thus, the formula mass of sodium chloride is atomic mass of sodium + atomic mass of chlorine
1.8 MOLE CONCEPT AND MOLAR MASSES
Atoms and molecules are extremely small in size and their numbers in even a small amount of any substance is really very large. To handle such large numbers, a unit of convenient magnitude is required.
Just as we denote one dozen for 12 items, score for 20 items, gross for 144 items, we use the idea of mole to count entities at the microscopic level (i.e., atoms, molecules, particles, electrons, ions, etc).
In SI system, mole (symbol, mol) was introduced as seventh base quantity for the amount of a substance.
The mole, symbol mol, is the SI unit of amount of substance. One mole contains exactly
This number of entities in
602213670000000000000000
Hence, so many entities (atoms, molecules or any other particle) constitute one mole of a particular substance.
We can, therefore, say that
1 mol of sodium chloride
Having defined the mole, it is easier to know the mass of one mole of a substance or the constituent entities. The mass of one mole of a substance in grams is called its molar mass. The molar mass in grams is numerically equal to atomic/molecular/ formula mass in
Molar mass of water
Molar mass of sodium chloride
1.9 PERCENTAGE COMPOSITION
So far, we were dealing with the number of entities present in a given sample. But many a time, information regarding the percentage of a particular element present in a compound is required. Suppose, an unknown or new compound is given to you, the first question

you would ask is: what is its formula or what are its constituents and in what ratio are they present in the given compound? For known compounds also, such information provides a check whether the given sample contains the same percentage of elements as present in a pure sample. In other words, one can check the purity of a given sample by analysing this data.
Let us understand it by taking the example of water
Mass % of an element
mass of that element in the compound
Molar mass of water
Mass
Mass % of oxygen
Let us take one more example. What is the percentage of carbon, hydrogen and oxygen in ethanol?
Molecular formula of ethanol is:
Molar mass of ethanol is:
Mass per cent of carbon
Mass per cent of hydrogen
Mass per cent of oxygen
After understanding the calculation of per cent of mass, let us now see what information can be obtained from the per cent composition data.
1.9.1 Empirical Formula for Molecular Formula
An empirical formula represents the simplest whole number ratio of various atoms present in a compound, whereas, the molecular formula shows the exact number of different types of atoms present in a molecule of a compound.
If the mass per cent of various elements present in a compound is known, its empirical formula can be determined. Molecular formula can further be obtained if the molar mass is known. The following example illustrates this sequence.
1.10 STOICHIOMETRY AND STOICHIOMETRIC CALCULATIONS
The word ‘stoichiometry’ is derived from two Greek words - stoicheion (meaning, element) and metron (meaning, measure). Stoichiometry, thus, deals with the calculation of masses (sometimes volumes also) of the reactants and the products involved in a chemical reaction. Before understanding how to calculate the amounts of reactants required or the products produced in a chemical reaction, let us study what information is available from the balanced chemical equation of a given reaction. Let us consider the combustion of methane. A balanced equation for this reaction is as given below:
Here, methane and dioxygen are called reactants and carbon dioxide and water are called products. Note that all the reactants and the products are gases in the above reaction and this has been indicated by letter
The coefficients 2 for
Thus, according to the above chemical reaction,
-
One mole of
-
One molecule of
-
22.7
-
From these relationships, the given data can be interconverted as follows: mass
mass
1.10.1 Limiting Reagent
Many a time, reactions are carried out with the amounts of reactants that are different than the amounts as required by a balanced chemical reaction. In such situations, one reactant is in more amount than the amount required by balanced chemical reaction. The reactant which is present in the least amount gets consumed after sometime and after that further reaction does not take place whatever be the amount of the other reactant. Hence, the reactant, which gets consumed first, limits the amount of product formed and is, therefore, called the limiting reagent.
In performing stoichiometric calculations, this aspect is also to be kept in mind.
1.10.2 Reactions in Solutions
A majority of reactions in the laboratories are carried out in solutions. Therefore, it is important to understand as how the amount of substance is expressed when it is present in the solution. The concentration of a solution or the amount of substance present in its given volume can be expressed in any of the following ways.
1. Mass per cent or weight per cent (w/w %)
2. Mole fraction
3. Molarity
4. Molality
Let us now study each one of them in detail.
Balancing a chemical equation
According to the law of conservation of mass, a balanced chemical equation has the same number of atoms of each element on both sides of the equation. Many chemical equations can be balanced by trial and error. Let us take the reactions of a few metals and non-metals with oxygen to give oxides
Equations (a) and (b) are balanced, since there are same number of metal and oxygen atoms on each side of the equations. However equation (c) is not balanced. In this equation, phosphorus atoms are balanced but not the oxygen atoms. To balance it, we must place the coefficient 5 on the left of oxygen on the left side of the equation to balance the oxygen atoms appearing on the right side of the equation.
Now, let us take combustion of propane,
Step 1 Write down the correct formulas of reactants and products. Here, propane and oxygen are reactants, and carbon dioxide and water are products.
Step 2 Balance the number of
Step 3 Balance the number of
Step 4 Balance the number of
Step 5 Verify that the number of atoms of each element is balanced in the final equation. The equation shows three carbon atoms, eight hydrogen atoms, and 10 oxygen atoms on each side.
All equations that have correct formulas for all reactants and products can be balanced. Always remember that subscripts in formulas of reactants and products cannot be changed to balance an equation.
1. Mass per cent
It is obtained by using the following relation:
Mass per cent
2. Mole Fraction
It is the ratio of number of moles of a particular component to the total number of moles of the solution. If a substance ’
3. Molarity
It is the most widely used unit and is denoted by
Molarity
Suppose, we have
Hence, for making
Now, how much volume of concentrated (1M)
If
then,
Thus,
In fact for such calculations, a general formula,
Note that the number of moles of solute
4. Molality
It is defined as the number of moles of solute present in
Thus, Molality
Summary
Chemistry, as we understand it today is not a very old discipline. People in ancient India, already had the knowledge of many scientific phenomenon much before the advent of modern science. They applied the knowledge in various walks of life.
The study of chemistry is very important as its domain encompasses every sphere of life. Chemists study the properties and structure of substances and the changes undergone by them. All substances contain matter, which can exist in three states solid, liquid or gas. The constituent particles are held in different ways in these states of matter and they exhibit their characteristic properties. Matter can also be classified into elements, compounds or mixtures. An element contains particles of only one type, which may be atoms or molecules. The compounds are formed where atoms of two or more elements combine in a fixed ratio to each other. Mixtures occur widely and many of the substances present around us are mixtures.
When the properties of a substance are studied, measurement is inherent. The quantification of properties requires a system of measurement and units in which the quantities are to be expressed. Many systems of measurement exist, of which the English and the Metric Systems are widely used. The scientific community, however, has agreed to have a uniform and common system throughout the world, which is abbreviated as SI units (International System of Units).
Since measurements involve recording of data, which are always associated with a certain amount of uncertainty, the proper handling of data obtained by measuring the quantities is very important. The measurements of quantities in chemistry are spread over a wide range of
The combination of different atoms is governed by basic laws of chemical combination - these being the Law of Conservation of Mass, Law of Definite Proportions, Law of Multiple Proportions, Gay Lussac’s Law of Gaseous Volumes and Avogadro Law. All these laws led to the Dalton’s atomic theory, which states that atoms are building blocks of matter. The atomic mass of an element is expressed relative to
The number of atoms, molecules or any other particles present in a given system are expressed in the terms of Avogadro constant
Chemical reactions represent the chemical changes undergone by different elements and compounds. A balanced chemical equation provides a lot of information. The coefficients indicate the molar ratios and the respective number of particles taking part in a particular reaction. The quantitative study of the reactants required or the products formed is called stoichiometry. Using stoichiometric calculations, the amount of one or more reactant(s) required to produce a particular amount of product can be determined and vice-versa. The amount of substance present in a given volume of a solution is expressed in number of ways, e.g., mass per cent, mole fraction, molarity and molality.






