Chemical Reactions and Equations
Question 1: Why should a magnesium ribbon be cleaned before burning in air?
Answer
Magnesium is very reactive metal. When stored it reacts with oxygen to form a layer magnesium oxide on its surface. This layer of magnesium oxide is quite stable and prevents further reaction of magnesium with oxygen. The magnesium ribbon is cleaned by sand paper to remove this layer so that the underlying metal can be exposed into air.
Question 2: Write the balanced equation for the following chemical reactions.
(i) Hydrogen + Chlorine
(ii) Barium chloride + Aluminium sulphate
(iii) Sodium + Water
Answer
(i)
(ii)
(iii)
Question 3: Write a balanced chemical equation with state symbols for the following reactions.
(i) Solutions of barium chloride and sodium sulphate in water react to give insoluble barium sulphate and the solution of sodium chloride.
(ii) Sodium hydroxide solution (in water) reacts with hydrochloric acid solution (in water) to produce sodium chloride solution and water.
Answer
$ \begin{aligned} &(i) ~BaCl_{2(a q)}+Na_2 SO _{4(a q)} \longrightarrow BaSO _{4(s)}+2 NaCl {(a q)} \ & (ii) ~NaOH{(a q)}+HCl _{(a q)} \longrightarrow NaCl _{(a q)}+H_2 O _{(j)} \end{aligned} $
Question 4: A solution of a substance ’
(i) Name the substance ’
(ii) Write the reaction of the substance ’
**Answer
**: The substance ’
Calcium oxide Water Calcium hydroxide
(Quick lime)
(Slaked lime)
Question 5: Why is the amount of gas collected in one of the test tubes in Activity 1.7 double of the amount collected in the other? Name this gas.
Answer
Water (H2O) contains two parts hydrogen and one part oxygen. Therefore, the amount of hydrogen and oxygen produced during electrolysis of water is in a 2:1 ratio. During electrolysis, since hydrogen goes to one test tube and oxygen goes to another, the amount of gas collected in one of the test tubes is double of the amount collected in the other.
Question 6: Why does the colour of copper sulphate solution change when an iron nail is dipped in it?
Answer
When an iron nail is placed in a copper sulphate solution, iron displaces copper from copper sulphate solution forming iron sulphate, which is green in colour.

Therefore, the blue colour of copper sulphate solution fades and green colour appears.
Question 7: Give an example of a double displacement reaction other than the one given in Activity 1.10. Sodium carbonate reacts with calcium chloride to form calcium carbonate and sodium chloride.
Answer
| Sodium | Calcium | Calcium | ||
| carbonate | chloride | carbonate | Sodium | |
| chloride |
In this reaction, sodium carbonate and calcium chloride exchange ions to form two new compounds. Hence, it is a double displacement reaction.
Question 8: Identify the substances that are oxidised and the substances that are reduced in the following reactions.
Answer
(i) Sodium (Na) is oxidised as it gains oxygen and oxygen gets reduced.
(ii) Copper oxide
Question 9: Which of the statements about the reaction below are incorrect?
(a) Lead is getting reduced. (b) Carbon dioxide is getting oxidised. (c) Carbon is getting oxidised.
(d) Lead oxide is getting reduced.
(i) (a) and (b)
(ii) (a) and (c)
(iii) (a), (b) and (c)
(iv) all
Answer
(i) (a) and (b)
Question 10:
The above reaction is an example of a
(a) combination reaction.
(b) double displacement reaction.
(c) decomposition reaction.
(d) displacement reaction
Answer
(d) The given reaction is an example of a displacement reaction.
Question 11: What happens when dilute hydrochloric acid is added to iron filings? Tick the correct Answer
.
(a) Hydrogen gas and iron chloride are produced.
(b) Chlorine gas and iron hydroxide are produced.
(c) No reaction takes place.
(d) Iron salt and water are produced.
Answer
(a) Hydrogen gas and iron chloride are produced. The reaction is as follows:
Question 12: What is a balanced chemical equation? Why should chemical equations be balanced?
Answer
A reaction which has an equal number of atoms of all the elements on both sides of the chemical equation is called a balanced chemical equation. The law of conservation of mass states that mass can neither be created nor destroyed. Hence, in a chemical reaction, the total mass of reactants should be equal to the total mass of the products. It means that the total number of atoms of each element should be equal on both sides of a chemical equation. Hence, it is for this reason that chemical equations should be balanced.
Question 13: Translate the following statements into chemical equations and then balance them.
(a) Hydrogen gas combines with nitrogen to form ammonia.
(b) Hydrogen sulphide gas burns in air to give water and sulphur dioxide.
(c) Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.
(d) Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.
Answer
(a)
(b)
(c)

(d)
Question 14: Balance the following chemical equations.
(a)

(b)
(c)
(d)
Answer
(a)
(b)
(c)
(d)
Question 15: Write the balanced chemical equations for the following reactions.
(a) Calcium hydroxide + Carbon dioxide
(b) Zinc + Silver nitrate
(c) Aluminium + Copper chloride
(d) Barium chloride + Potassium sulphate
Answer
(a)
(b)
(c)
(d)
Question 16: Write the balanced chemical equation for the following and identify the type of reaction in each case.
(a)Potassium bromide (aq) + Barium iodide (aq)
(b) Zinc carbonate (s)
(c) Hydrogen (g) + Chlorine (g)
(d) Magnesium (s) + Hydrochloric acid (aq)
Answer
(a)
(b)
(c)
(d)

Question 17: What does one mean by exothermic and endothermic reactions? Give examples.
Answer
Chemical reactions that release energy in the form of heat, light, or sound are called exothermic reactions.
Example: Mixture of sodium and chlorine to yield table salt
In other words, combination reactions are exothermic.
Reactions that absorb energy or require energy in order to proceed are called endothermic reactions.
For example: In the process of photosynthesis, plants use the energy from the sun to convert carbon dioxide and water to glucose and oxygen.
Glucose
Question 18: Why is respiration considered an exothermic reaction? Explain.
Answer
Energy is required to support life. Energy in our body is obtained from the food we eat. During digestion, large molecules of food are broken down into simpler substances such as glucose. Glucose combines with oxygen in the cells and provides energy. The special name of this combustion reaction is respiration. Since energy is released in the whole process, it is an exothermic process.

Glucose Oxygen Carbondioxide Water
Question 19: Why are decomposition reactions called the opposite of combination reactions? Write equations for these reactions.
Answer
Decomposition reactions are those in which a compound breaks down to form two or more substances. These reactions require a source of energy to proceed. Thus, they are the exact opposite of combination reactions in which two or more substances combine to give a new substance with the release of energy.
Decomposition reaction:
Combination reaction:
Write one equation each for decomposition reactions where energy is supplied in the form of heat, light or electricity.
(a) Thermal decomposition:
Ferrous sulphate Ferric oxide Sulphur dioxide Sulphur trioxide
(b) Decomposition by light:

(c) Decomposition by electricity:
Aluminium oxide Aluminium Oxygen
Question 20: What is the difference between displacement and double displacement reactions? Write equations for these reactions.
Answer
In a displacement reaction, a more reactive element replaces a less reactive element from a compound.
B
In a double displacement reaction, two atoms or a group of atoms switch places to form new compounds.
For example:
Displacement reaction:
Double displacement reaction:
Question 21: In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. Write down the reaction involved.
Answer
Silvernitrate Copper Coppernitrate
Question 22: What do you mean by a precipitation reaction? Explain by giving examples.
Answer
A reaction in which an insoluble solid (called precipitate) is formed is called a precipitation reaction.
For example:
Sodium carbonate Calcium chloride Calcium carbonate Sodium chloride
In this reaction, calcium carbonate is obtained as a precipitate. Hence, it is a precipitation reaction.
Another example of precipitation reaction is:
 Sodium sulphate Barium chloride Barium sulphate Sodium chloride
Sodium sulphate Barium chloride Barium sulphate Sodium chloride
In this reaction, barium sulphate is obtained as a precipitate.
Question 23: Explain the following in terms of gain or loss of oxygen with two examples each.
(a) Oxidation
(b) Reduction
(a) Oxidation is the gain of oxygen.
Answer
For example: (i)
(ii)

In equation (i),
(b) Reduction is the loss of oxygen.
For example:
(i)

(ii)
In equation (i),
Question 24: A shiny brown-coloured element ’
Question 25: Why do we apply paint on iron articles?
Answer
Iron articles are painted because it prevents them from rusting. When painted, the contact of iron articles from moisture and air is cut off. Hence, rusting is prevented their presence is essential for rusting to take place.
Question 26: Oil and fat containing food items are flushed with nitrogen. Why?
Answer
Nitrogen is an inert gas and does not easily react with these substances. On the other hand, oxygen reacts with food substances and makes them rancid. Thus, bags used in packing food items are flushed with nitrogen gas to remove oxygen inside the pack. When oxygen is not present inside the pack, rancidity of oil and fat containing food items is avoided.
Question 27: Explain the following terms with one example each.
(a) Corrosion
(b) Rancidity
(a) Corrosion:
Answer
(a) Corrosion: Corrosion is defined as a process where materials, usually metals, deteriorate as a result of a chemical reaction with air, moisture, chemicals, etc.
For example, iron, in the presence of moisture, reacts with oxygen to form hydrated iron oxide.
This hydrated iron oxide is rust.
(b) Rancidity:
The process of oxidation of fats and oils that can be easily noticed by the change in taste and smell is known as rancidity.
For example, the taste and smell of butter changes when kept for long.
Rancidity can be avoided by:
-
Storing food in air tight containers
-
Storing food in refrigerators
-
Adding antioxidants
-
Storing food in an environment of nitrogen
Acids, Bases and Salts
Question 1: You have been provided with three test tubes. One of them contains distilled water and the other two contain an acidic solution and a basic solution, respectively. If you are given only red litmus paper, how will you identify the contents of each test tube?
Solution:- If the colour of red litmus paper gets changed to blue, then it is a base and if there is no colour change, then it is either acidic or neutral. Thus, basic solution can be easily identified.
Let us mark the three test tubes as A, B, and C. A drop of the solution in A is put on the red litmus paper. Same is repeated with solution
Question 2: Why should curd and sour substances not be kept in brass and copper vessels?
Answer Curd and other sour substances contain acids. Therefore, when they are kept in brass and copper vessels, the metal reacts with the acid to liberate hydrogen gas and harmful products, thereby spoiling the food.
Question 3: Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas?
Solution:- Hydrogen gas is usually liberated when an acid reacts with a metal.

Take few pieces of zinc granules and add
We can test the evolved hydrogen gas by its burning with a pop sound when a candle is brought near the soap bubbles.
Question 4: Metal compound A reacts with dilute hydrochloric acid to produce effervescence.
The gas evolved extinguishes a burning candle. Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride.
Answer

Calcium Hydrochloricacid Calcium chloride Carbon dioxide Water
carbonate
Question 5: Why do
Answer
The dissociation of
The reaction is as follows:
Although aqueous solutions of glucose and alcohol contain hydrogen, these cannot dissociate in water to form hydrogen ions. Hence, they do not show acidic character.
Question 6: Why does an aqueous solution of an acid conduct electricity?
Answer
Acids dissociate in aqueous solutions to form ions. These ions are responsible for conduction of electricity.
Question 7: Why does dry HCl gas not change the colour of the dry litmus paper?
Answer
Colour of the litmus paper is changed by the hydrogen ions. Dry HCl gas does not contain
Since the process of dissolving an acid in water is exothermic, it is always recommended that acid should be added to water. If it is done the other way, then it is possible that because of the large amount of heat generated, the mixture splashes out and causes burns.
Question 8: How is the concentration of hydronium ions (H3O+) affected when a solution of an acid is diluted?
Answer
When an acid is diluted, the concentration of hydronium ions (
Question 9: How is the concentration of hydroxide ions
Answer
The concentration of hydroxide ions
Question 10: You have two solutions, A and B. The
Answer
A pH value of less than 7 indicates an acidic solution, while greater than 7 indicates a basic solution. Therefore, the solution with
Question 11: What effect does the concentration of
Answer
Concentration of
Question 12: Do basic solutions also have
Answer
Yes, basic solution also has
Question 13: Under what soil condition do you think a farmer would treat the soil of his fields with quick lime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate) ?
Answer
If the soil is acidic and improper for cultivation, then to increase the basicity of soil, the farmer would treat the soil with quick lime or slaked lime or chalk.
Question 14: What is the common name of the compound
Answer
The common name of the compound
Question 15: Name the substance which on treatment with chlorine yields bleaching powder?
Answer
Calcium hydroxide [Ca
Question 16: Name the sodium compound which is used for softening hard water.
Answer
Washing soda (Na2CO3.10H2O) is used for softening hard water.
Question 17:- What will happen if a solution of sodium hydrocarbonate is heated? Give the equation of the reaction involved.
Answer
When a solution of sodium hydrocarbonate (sodium hydrogencarbonate) is heated, sodium carbonate and water are formed with the evolution of carbon dioxide gas.

Question 18: Write an equation to show the reaction between Plaster of Paris and water.
Answer
The chemical equation for the reaction of Plaster of Paris and water can be represented as
Plaster of Paris Water Gypsum
Question 19: A solution turns red litmus blue, its
(a) 1 (b) 4 (c) 5 (d) 10
Answer
(d) Bases turn red litmus blue and acids turn blue litmus red. Basic solution has a pH value more than 7 . Since the solution turns red litmus blue, its
Question 20: A solution reacts with crushed egg-shells to give a gas that turns lime-water milky. The solution contains
(a)
(b)
(c)
(d)
Answer
(b) The solution contains
Question 21:
(a)
(b)
(c)
(d)
Answer
(d)
Question 22: Which one of the following types of medicines is used for treating indigestion?
(a) Antibiotic
(b) Analgesic
(c) Antacid
(d) Antiseptic
Answer
(c) Antacid is used for treating indigestion.
Question 23: Write word equations and then balanced equations for the reaction taking place when -
(a) dilute sulphuric acid reacts with zinc granules.
(b) dilute hydrochloric acid reacts with magnesium ribbon.
(c) dilute sulphuric acid reacts with aluminium powder.
(d) dilute hydrochloric acid reacts with iron filings.
Answer
(a) Sulphuric acid + Zinc
(b) Hydrochloric acid + Magnesium
(c) Sulphuric acid + Aluminium
(d) Hydrochloric acid + Iron
Question 24: Compounds such as alcohols and glucose also contain hydrogen but are not categorized as acids. Describe an activity to prove it.
Answer
Two nails are fitted on a cork and are kept it in a
Observations:
It will be observed that the bulb glows in the 
Result:
Conclusion:
From this activity, it can be concluded that all acids contain hydrogen but not all compounds containing hydrogen are acids.
That is why, though alcohols and glucose contain hydrogen, they are not categorised as acids.
Question 25: Why does distilled water not conduct electricity, whereas rain water does?
Answer
Distilled water is a pure form of water and is devoid of any ionic species. Therefore, it does not conduct electricity. Rain water, being an impure form of water, contains many ionic species such as acids and therefore it conducts electricity.
Question 26: Why do acids not show acidic behaviour in the absence of water?
Acids do not show acidic behaviour in the absence of water because the dissociation of hydrogen ions from an acid occurs in the presence of water only. It is the hydrogen ions that are responsible for the acidic behaviour.
Question 27: Five solutions A, B, C, D and E when tested with universal indicator showed
(b) strongly alkaline?
(c) strongly acidic?
(d) weakly acidic?
(e) weakly alkaline?
Answer
Arrange the pH in increasing order of hydrogen-ion concentration.
(a) Neutral
(b) Strongly alkaline
(c) Strongly acidic
(d) Weakly acidic
(e) Weakly alkaline
The
Question 28: Equal lengths of magnesium ribbons are taken in test tubes A and B. Hydrochloric acid
Answer
The fizzing will occur strongly in test tube A, in which hydrochloric acid (
Question 29: Fresh milk has a pH of 6. How do you think the pH will change as it turns into curd? Explain your
Answer
The pH of milk is 6 . As it changes to curd, the
Question 30: A milkman adds a very small amount of baking soda to fresh milk.
(a) Why does he shift the
(b) Why does this milk take a long time to set as curd?
Answer
(a) The milkman shifts the
(b) Since this milk is slightly basic than usual milk, acids produced to set the curd are neutralized by the base. Therefore, it takes a longer time for the curd to set.
Question 31: Plaster of Paris should be stored in a moisture-proof container. Explain why?
Answer
Plaster of Paris (POP) should be stored in a moisture-proof container because Plaster of Paris, a powdery mass, absorbs water (moisture) to form a hard solid known as gypsum.
Question 32: What is a neutralization reaction? Give two examples.
Answer
A reaction in which an acid and base react with each other to give a salt and water is termed as neutralization reaction. In this reaction, energy is evolved in the form of heat.
For example:
(i)
(ii) During indigestion (caused due to the production of excess of hydrochloric acid in the stomach), we administer an antacid (generally milk of magnesia,
Question 33: Give two important uses of washing soda and baking soda.
Answer
Two important used of washing soda and baking soda are as follows:
(1) Washing soda:
(a) It is used in glass, soap, and paper industries.
(b) It is used to remove permanent hardness of water.
(2) Baking soda:
(a) It is used as baking powder. Baking powder is a mixture of baking soda and a mild acid known as tartaric acid. When it is heated or mixed in water, it releases CO2 that makes bread or cake fluffy.
(b) It is used in soda-acid fire extinguishers.
Metals and Non-metals
Question 1: Explain the meanings of malleable and ductile.
Answer
Malleable: Substances that can be beaten into thin sheets are called malleable. For example, most of the metals are malleable.
Ductile: Substances that can be drawn into thin wires are called ductile. For example, most of the metals are ductile.
Question 2: Why is sodium kept immersed in kerosene oil?
Answer
Sodium and potassium are very reactive metals and and combine explosively with air as well as water. Hence, they catch fire if kept in open. Therefore, to prevent accidental fires and accidents, sodium is stored immersed in kerosene oil.
Question 3: Write equations for the reactions of
(i) iron with steam
(ii) calcium and potassium with water
Answer
(i)
(ii)
Question 4: Samples of four metals A, B, C and D were taken and added to the following solution one by one. The results obtained have been tabulated as follows.
MetalIron (II) sulphateCooper (II) sulphateZinc sulphate Silver nitrate
A. No reaction Displacement
B. Displacement No reaction
C. No reaction No reaction No reaction Displacement
D. No reaction No reaction No reaction No reaction
Use the Table above to Answer the following questions about metals A, B, C and D.
(i) Which is the most reactive metal?
(ii) What would you observe if B is added to a solution of copper (II) sulphate?
(iii) Arrange the metals A, B, C and D in the order of decreasing reactivity.
Answer
Explanation
From the above equations, we obtain:

(i)
(ii) If B is added to a solution of copper (II) sulphate, then it would displace copper.
B + CuSO4
(iii) The arrangement of the metals in the order of decreasing reactivity is:
Question 5: Which gas is produced when dilute hydrochloric acid is added to a reactive metal? Write the chemical reaction when iron reacts with dilute
Answer
Hydrogen gas is evolved when dilute hydrochloric acid is added to a reactive metal.
When iron reacts with dilute H2SO4, iron (II) sulphate with the evolution of hydrogen gas is formed.
Question 6: What would you observe when zinc is added to a solution of iron (II) sulphate? Write the chemical reaction that takes place.
Answer
Zinc is more reactive than iron. Therefore, if zinc is added to a solution of iron (II) sulphate, then it would displace iron from the solution.
Question 7: (i) Write the electron-dot structures for sodium, oxygen and magnesium.
(ii) Show the formation of
(iii) What are the ions present in these compounds?
Answer
(i) The representation of elements with valence electrons as dots around the elements is referred to as electron-dot structure for elements.
(a)
(b) Oxygen
(c) Magnesium
(ii)


(iii) The ions present in
Question 8: Why do ionic compounds have high melting points?
Answer
Ionic compounds have strong electrostatic forces of attraction between the ions. Therefore, it requires a lot of energy to overcome these forces. That is why ionic compounds have high melting points.
Question 9: Define the following terms.
(i) Mineral (ii) Ore (iii) Gangue
Answer
(i) Mineral: Most of the elements occur in nature as in combined state as minerals. The chemical composition of minerals is fixed.
(ii) Ore: Minerals from which metals can be extracted profitably are known as ores.
(iii) Gangue: The impurities (sand, silt, soil, gravel, etc.) present in the ore are called gangue.
Question 10: Name two metals which are found in nature in the free state.
The metals at the bottom of the reactivity series are mostly found in free state. For example: gold, silver, and platinum.
Question 11: What chemical process is used for obtaining a metal from its oxide?
Answer
The chemical process used for obtaining a metal from its oxide is reduction. In this process, metal oxides are reduced by using suitable reducing agents such as carbon or by highly reactive metals to displace the metals from their oxides.
For example, zinc oxide is reduced to metallic zinc by heating with carbon.
Manganese dioxide is reduced to manganese by treating it with aluminium powder. In this case, aluminium displaces manganese from its oxide.
Oxides of more reactive metals are reduced by electrolysis.
Question 12:
Define the following terms.
(i) Mineral
(ii) Ore
(iii) Gangue
Answer
(i) Mineral
The naturally occurring elements or compounds in the earth’s crust are known as minerals.
(ii) Ore Some minerals contain a large amount of a particular metal and metals can be extracted from them profitably. These minerals are known as ores.
(iii) Gangue : The impurities (sand, silt, soil, gravel, etc.) present in the ore are called gangue.
Question 13: Metallic oxides of zinc, magnesium and copper were heated with the following metals.
Metal ZincMagnesiumCopper
Zinc oxide
Magnesium oxide
Copper oxide
In which cases will you find displacement reactions taking place?
Answer
Metal Zinc Magnesium Copper
Zinc oxide No reaction DisplacementNo reaction
Magnesium oxide No reaction No reaction No reaction
Copper oxide DisplacementDisplacementNo reaction
Question 14: Which metals do not corrode easily?
Answer
More reactive a metal is, more likely it is to be corroded. Therefore, less reactive metals are less likely to get corroded. This is why gold plating provides high resistance to corrosion.
Question 15: What are alloys?
Answer
Alloys are homogeneous mixtures of two or more elements. The elements could be two metals, or a metal and a non-metal. An alloy is formed by first melting the metal and then dissolving the other elements in it. For example, steel is an alloy of iron and carbon.
Question 16: Which of the following pairs will give displacement reactions?
(a)
(b)
(c) FeSO4 solution and silver metal
(d) AgNO3 solution and copper metal.
(d) AgNO3 solution and copper metal
Question 17: Which of the following methods is suitable for preventing an iron frying pan from rusting?
(a) Applying grease
(b) Applying paint
(c) Applying a coating of zinc
(d) all of the above.
Answer
(c) Applying a coating of zinc
(We can also apply grease and paint to prevent iron from rusting. However, in case of iron frying pan, grease and paint cannot be applied because when the pan will be heated and washed again and again, the coating of grease and paint would get destroyed.)
Question 18: An element reacts with oxygen to give a compound with a high melting point. This compound is also soluble in water. The element is likely to be
(a) calcium
(b) carbon
(c) silicon
(d) iron
Answer
(a) The element is likely to be calcium
Question 19: Food cans are coated with tin and not with zinc because
(a) zinc is costlier than tin.
(b) zinc has a higher melting point than tin.
(c) zinc is more reactive than tin.
(d) zinc is less reactive than tin.
Answer
(c) Food cans are coated with tin and not with zinc because zinc is more reactive than tin.
Question 20: You are given a hammer, a battery, a bulb, wires and a switch.
(a) How could you use them to distinguish between samples of metals and non-metals?
(b) Assess the usefulness of these tests in distinguishing between metals and non-metals.
Answer
(a) With the hammer, we can beat the sample and if it can be beaten into thin sheets (that is, it is malleable), then it is a metal otherwise a non-metal. Similarly, we can use the battery, bulb, wires, and a switch to set up a circuit with the sample. If the sample conducts electricity, then it is a metal otherwise a non-metal.
(b) The above tests are useful in distinguishing between metals and non-metals as these are based on the physical properties. No chemical reactions are involved in these tests.
Question 21: What are amphoteric oxides? Give two examples of amphoteric oxides.
Those oxides that behave as both acidic and basic oxides are called amphoteric oxides.
Examples: aluminium oxide (Al2O3), zinc oxide (ZnO)
Question 22: Name two metals which will displace hydrogen from dilute acids, and two metals which will not.
Answer
Metals that are more reactive than hydrogen displace it from dilute acids. For example: sodium and potassium. Metals that are less reactive than hydrogen do not displace it. For example: copper and silver.
Question 23: In the electrolytic refining of a metal M, what would you take as the anode, the cathode and the electrolyte?
Answer
In the electrolytic refining of a metal M:
Anode
Cathode
Electrolyte
Question 24: Pratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it, as shown in figure below.

(a) What will be the action of gas on
(i) dry litmus paper?
(ii) moist litmus paper?
(b) Write a balanced chemical equation for the reaction taking place.
Answer
(a) (i) There will be no action on dry litmus paper.
(ii) Since the gas is sulphur dioxide (SO2), it turns moist blue litmus paper to red because sulphur dioxide reacts with moisture to form sulphurous acid.
(b)
Question 24: State two ways to prevent the rusting of iron.
Answer
Two ways to prevent the rusting of iron are:
(i) Oiling, greasing, or painting: By applying oil, grease, or paint, the surface becomes water proof and the moisture and oxygen present in the air cannot come into direct contact with iron. Hence, rusting is prevented.
(ii) Galvanisation: An iron article is coated with a layer of zinc metal, which prevents the iron to come in contact with oxygen and moisture. Hence, rusting is prevented.
Question 25: What type of oxides is formed when non-metals combine with oxygen?
Answer
Non-metals combine with oxygen to form acidic oxides.
For example:
(Acidic in nature)
Question 26: Give reasons
(a) Platinum, gold and silver are used to make jewellery.
(b) Sodium, potassium and lithium are stored under oil.
(c) Aluminium is a highly reactive metal, yet it is used to make utensils for cooking.
(d) Carbonate and sulphide ores are usually converted into oxides during the process of extraction.
Answer
(a) Platinum, gold, and silver are used to make jewellery because they are very lustrous. Also, they are very less reactive and do not corrode easily.
(b) Sodium, potassium, and lithium are very reactive metals and react very vigorously with air as well as water. Therefore, they are kept immersed in kerosene oil in order to prevent their contact with air and moisture.
(c) Though aluminium is a highly reactive metal, it is resistant to corrosion. This is because aluminium reacts with oxygen present in air to form a thin layer of aluminium oxide. This oxide layer is very stable and prevents further reaction of aluminium with oxygen. Also, it is light in weight and a good conductor of heat. Hence, it is used to make cooking utensils.
(d) Carbonate and sulphide ores are usually converted into oxides during the process of extraction because metals can be easily extracted from their oxides rather than from their carbonates and sulphides
Question 27: You must have seen tarnished copper vessels being cleaned with lemon or tamarind juice. Explain why these sour substances are effective in cleaning the vessels.
Answer
Copper reacts with moist carbon dioxide in air to form copper carbonate and as a result, copper vessel loses its shiny brown surface forming a green layer of copper carbonate. The citric acid present in the lemon or tamarind neutralises the basis copper carbonate and dissolves the layer. That is why, tarnished copper vessels are cleaned with lemon or tamarind juice to give the surface of the copper vessel its characteristic lustre.
Question 28: Differentiate between metal and non-metal on the basis of their chemical properties.
Answer
Metal
Metals are electropositive.
They react with oxygen to form basic oxides.
These have ionic bonds.
These have covalent bonds.
They react with water to form oxides and hydroxides. SomeThey do not react with water. metals react with cold water, some with hot water, and some with steam.
They react with dilute acids to form a salt and evolve hydrogen gas. However,
They react with the salt solution of metals. Depending on their reactivity, displacement reaction can occur.
They act as reducing agents (as they can easily lose electrons). They do not react with dilute acids. These are not capable of replacing hydrogen.
These react with the salt solution of non-metals.
These act as oxidising agents (as they can gain electrons).
Question 29: A man went door to door posing as a goldsmith. He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a set of gold bangles to him which he dipped in a particular solution. The bangles sparkled like new but their weight was reduced drastically. The lady was upset but after a futile argument the man beat a hasty retreat. Can you play the detective to find out the nature of the solution he had used?
Answer
He must have dipped the gold metal in the solution of aqua regia - a 3:1 mixture of conc.
Question 30: Give reasons why copper is used to make hot water tanks and not steel (an alloy of iron).
Answer
Copper does not react with cold water, hot water, or steam. However, iron reacts with steam. If the hot water tanks are made of steel (an alloy of iron), then iron would react vigorously with the steam formed from hot water.

That is why copper is used to make hot water tanks, and not steel.
Carbon and its Compounds
Question 1: What would be the electron dot structure of carbon dioxide which has the formula
Answer
Electron dot structure of

Question 2: What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur? (Hint - the eight atoms of sulphur are joined together in the form of a ring.)
Answer
Electron dot structure of a sulphur molecule

Question 3: How many structural isomers can you draw for pentane?
Answer
Three structural isomers are possible for pentane.
(i)
(ii)
(iii)
Question 4: What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Answer
The two features of carbon that give rise to a large number of compounds are as follows: (i) Catenation - It is the ability to form bonds with other atoms of carbon.
(ii) Tetravalency - With the valency of four, carbon is capable of bonding with four other atoms.
Question 5: What will be the formula and electron dot structure of cyclopentane?
Answer
The formula for cyclopentane is C5H10. Its electron dot structure is given below.

Question 6: Draw the structures for the following compounds.
(i) Ethanoic acid (ii) Bromopentane*
(iii) Butanone (iv) Hexanal
Answer
*Are structural isomers possible for bromopentane?
(i)
(ii) There are many structural isomers possible for bromopentane. Among them, the structures of three isomers are given.
(iii)
(iv)
Question 7: How would you name the following compounds?
(i)
(ii)
(iii)
Answer
(i) Bromoethane
(ii) Methanal (formaldehyde)
(iii) Hexyne
Question 8: Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
Ethanol Ethanoic acid
Answer
Since the conversion of ethanol to ethanoic acid involves the addition of oxygen to ethanol, it is an oxidation reaction.
Question 9: A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Answer
When ethyne is burnt in air, it gives a sooty flame. This is due to incomplete combustion caused by limited supply of air. However, if ethyne is burnt with oxygen, it gives a clean flame with temperature
Question 10: How would you distinguish experimentally between an alcohol and a carboxylic acid?
Answer
We can distinguish between an alcohol and a carboxylic acid on the basis of their reaction with carbonates and hydrogen carbonates. Acid reacts with carbonate and hydrogen carbonate to evolve
Metal Carbonate/ Metal Hydrogencarbonate + Carboxylic acid
Alcohols, on the other hand, do not react with carbonates and hydrogen carbonates.
Question 11: Would you be able to check if water is hard by using a detergent?
Answer
Detergents are ammonium or sulphonate salts of long chain carboxylic acids. Unlike soap, they do not react with calcium and magnesium ions present in hard water to form scum. They give a good amount of lather irrespective of whether the water is hard or soft. This means that detergents can be used in both soft and hard water. Therefore, it cannot be used to check whether the water is hard or not.
Question 12: People use a variety of methods to wash clothes. Usually after adding the soap, they ‘beat’ the clothes on a stone, or beat it with a paddle, scrub with a brush or the mixture is agitated in a washing machine. Why is agitation necessary to get clean clothes?
Answer
A soap molecule has two parts namely hydrophobic and hydrophilic. With the help of these, it attaches to the grease or dirt particle and forms a cluster called micelle. These micelles remain suspended as a colloid. To remove these micelles (entrapping the dirt), it is necessary to agitate clothes.
Question 13: Ethane, with the molecular formula C2H6 has
(a) 6 covalent bonds.
(b) 7 covalent bonds.
(c) 8 covalent bonds.
(d) 9 covalent bonds.
Answer
(b) Ethane has 7 covalent bonds.
Question 14: Butanone is a four-carbon compound with the functional group
(a) carboxylic acid.
(b) aldehyde.
(c) ketone.
(d) alcohol.
Answer
(c) The functional group of butanone is ketone.
Question 15: While cooking, if the bottom of the vessel is getting blackened on the outside, it means that
(a) the food is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the fuel is burning completely.
Answer
(b) While cooking, if the bottom of the vessel is getting blackened on the outside, then it means that the fuel is not burning completely.
Question 16: Explain the nature of the covalent bond using the bond formation in
Answer
Carbon can neither lose four of its electrons nor gain four electrons as both the processes require extra amount of energy and would make the system unstable. Therefore, it completes its octet by sharing its four electrons with other carbon atoms or with atoms of other elements. The bonds that are formed by sharing electrons are known as covalent bonds. In covalent bonding, both the atoms share the valence electrons, i.e., the shared electrons belong to the valence shells of both the atoms.

Here, carbon requires 4 electrons to complete its octet, while each hydrogen atom requires one electron to complete its duplet. Also, chlorine requires an electron to complete the octet. Therefore, all of these share the electrons and as a result, carbon forms 3 bonds with hydrogen and one with chlorine.
Question 17: Draw the electron dot structures for
(a) ethanoic acid.
(b) H2S.
(c) propanone.
(d) F2.
Answer
(a) Ethanoic acid

(b) H2 S

(c) Propanone

(d) F2
Question 18: What is a homologous series? Explain with an example.
Answer
A homologous series is a series of carbon compounds that have different numbers of carbon atoms but contain the same functional group.
For example, methane, ethane, propane, butane, etc. are all part of the alkane homologous series. The general formula of this series is
Methane CH4
Ethane
Propane
Butane
It can be noticed that there is a difference of -
Question 19: How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties?
Answer
Metal Carbonates/Metal Hydrogencarbonates + Carboxylic acid
For example,
Metal Carbonates/Metal Hydrogencarbonates + Alcohols

No reaction
For example,
Question 20: Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
Answer
A soap is a sodium or potassium salt of long chain fatty acids. It has one polar end and one non-polar end. The polar end is hydrophilic in nature i.e., this end is attracted towards water. The non-polar end is hydrophobic but lipophilic, i.e., it is attracted towards hydrocarbons. When soap is added to water, soap molecules arrange themselves in a cluster to keep the non-polar portion out of water such that the non-polar ends are in the interior of the cluster and the polar ends are on the surface of the cluster. Since the dirt present on clothes is organic in nature and insoluble in water, the hydrophobic ends of the clusters attach themselves to the dirt. This cluster formation in which the dirt is entrapped is the micelle.

Micelle formation does not occur in alcohol because the alkyl chain of soap becomes soluble in alcohol.
Question 21: Why are carbon and its compounds used as fuels for most applications?
Answer
Most of the carbon compounds give a lot of heat and light when burnt in air. Saturated hydrocarbons burn with a clean flame and no smoke is produced. The carbon compounds, used as a fuel, have high calorific values. Therefore, carbon and its compounds are used as fuels for most applications.
Question 22: Explain the formation of scum when hard water is treated with soap.
Answer
Soap does not work properly when the water is hard. A soap is a sodium or potassium salt of long chain fatty acids. Hard water contains salts of calcium and magnesium. When soap is added to hard water, calcium and magnesium ions present in water displace sodium or potassium ions from the soap molecules forming an insoluble substance called scum. A lot of soap is wasted in the process.
Question 23: What change will you observe if you test soap with litmus paper (red and blue) ?
Answer
Since soap is basic in nature, it will turn red litmus blue. However, the colour of blue litmus will remain blue.
Question 24: What is hydrogenation? What is its industrial application?
Answer
Hydrogenation is the process of addition of hydrogen. Unsaturated hydrocarbons are added with hydrogen in the presence of palladium and nickel catalysts to give saturated hydrocarbons.
This reaction is applied in the hydrogenation of vegetables oils, which contain long chains of unsaturated carbons.
Question 25: Which of the following hydrocarbons undergo addition reactions:
C2H6, C3H8, C3H6, C2H2 and CH4.
Answer
Unsaturated hydrocarbons undergo addition reactions. Being unsaturated hydrocarbons, C3H6 and C2H2undergo addition reactions.
Question 26: Give a test that can be used to differentiate chemically between butter and cooking oil.
Answer
Butter contains saturated fats. Therefore, it cannot be hydrogenated. On the other hand, oil has unsaturated fats. That is why it can be hydrogenated to saturated fats (solids).
Question 27: Explain the mechanism of the cleaning action of soaps.
Answer
Cleansing action of soaps:
The dirt present on clothes is organic in nature and insoluble in water. Therefore, it cannot be removed by only washing with water. When soap is dissolved in water, its hydrophobic ends attach themselves to the dirt and remove it from the cloth. Then, the molecules of soap arrange themselves in micelle formation and trap the dirt at the centre of the cluster. These micelles remain suspended in the water. Hence, the dust particles are easily rinsed away by water.

Periodic Classification of Elements
Question 1: Did Dobereiner’s triads also exist in the columns of Newlands’ Octaves? Compare and find out.
Answer
Only one triad of Dobereiner’s triads exists in the columns of Newlands’ octaves. The triad formed by the elements
Dobereiner’s triads
Newlands’ octaves
| H | Li Be B | C N O |
|---|---|---|
| F | NaMgAl | SiP S |
| Cl | K Ca Cr | TiMnFe |
| Co and NiCuZn Y | InAs Se | |
| Br | RbSr Ce and LaZr – |
Question 2: What were the limitations of Dobereiner’s classification?
Answer
Limitation of Dobereiner’s classification:
All known elements could not be classified into groups of triads on the basis of their properties.
Question 3: What were the limitations of Newlands’ Law of Octaves?
Answer
Limitations of Newlands’ law of octaves:
(i) It was not applicable throughout the arrangements. It was applicable up to calcium only. The properties of the elements listed after calcium showed no resemblance to the properties of the elements above them.
(ii) Those elements that were discovered after Newlands’ octaves did not follow the law of octaves.
(iii) The position of cobalt and nickel in the group of the elements (
(iv) Placing of iron far away from cobalt and nickel, which have similar properties as iron, could also not be explained.
Question 4: Use Mendeleev’s Periodic Table to predict the formulae for the oxides of the following elements:
K, C, Al, Si, Ba.
Answer
Ba is in group 2. Therefore, the oxide will be BaO.
Question 5: Besides gallium, which other elements have since been discovered that were left by Mendeleev in his Periodic Table? (any two)
Answer
Scandium and germanium
Question 6: What were the criteria used by Mendeleev in creating his Periodic Table?
Answer
Mendeleev’s periodic table was based on the observation that the properties of elements are a periodic function of their atomic masses. This means that if elements are arranged in the increasing order of their atomic masses, then their properties get repeated after regular intervals.
Question 7: Why do you think the noble gases are placed in a separate group?
Noble gases are inert elements. Their properties are different from the all other elements.
Therefore, the noble gases are placed in a separate group.
Question 8: How could the Modern Periodic Table remove various anomalies of Mendeleev’s Periodic Table?
Answer
Mendeleev was unable to give fixed position to hydrogen and isotopes in the periodic table. In Mendeleev’s periodic table, the increasing manner of atomic mass of the elements is not always regular from one to its next. It was believed that a more fundamental property than atomic mass could explain periodic properties in a better manner.
It was Henry Moseley who demonstrated that atomic number of an element could explain periodic properties in a better way than atomic mass of an element and arranged the elements in increasing order of their atomic numbers. Then it was found that the various anomalies of Mendeleev’s periodic table were removed by the modern periodic table.
Question 9: Name two elements you would expect to show chemical reactions similar to magnesium. What is the basis for your choice?
Answer
Calcium (Ca) and strontium (Sr) are expected to show chemical reactions similar to magnesium (Mg). This is because the number of valence electrons (2) is same in all these three elements. And since chemical properties are due to valence electrons, they show same chemical reactions.
Question 10: Name
(a) three elements that have a single electron in their outermost shells.
(b) two elements that have two electrons in their outermost shells.
(c) three elements with filled outermost shells.
Answer
(a) Lithium (Li), sodium (Na), and potassium (K) have a single electron in their outermost shells.
(b) Magnesium (Mg) and calcium (Ca) have two electrons in their outermost shells.
(c) Neon (Ne), argon (Ar), and xenon (Xe) have filled outermost shells.
Question 11:
(a) Lithium, sodium, potassium are all metals that react with water to liberate hydrogen gas. Is there any similarity in the atoms of these elements?
(b) Helium is an unreactive gas and neon is a gas of extremely low reactivity. What, if anything, do their atoms have in common?
Answer
(a) Yes. The atoms of all the three elements lithium, sodium, and potassium have one electron in their outermost shells.
(b) Both helium (He) and neon (
Question 12: In the Modern Periodic Table, which are the metals among the first ten elements?
Answer
Among the first ten elements, lithium (
Question 13: By considering their position in the Periodic Table, which one of the following elements would you expect to have maximum metallic characteristic?
Answer
Since Be lies to the extreme left hand side of the periodic table, Be is the most metallic among the given elements.
Question 14: Which of the following statements is not a correct statement about the trends when going from left to right across the periods of periodic Table.
(a) The elements become less metallic in nature.
(b) The number of valence electrons increases.
(c) The atoms lose their electrons more easily.
(d) The oxides become more acidic.
Answer
(c) The atoms lose their electrons more easily.
(On moving from left to right across the periods of the periodic table, the non-metallic character increases. Hence, the tendency to lose electrons decreases.)
Question 15: Element X forms a chloride with the formula XCl2, which is a solid with a high melting point.
(c)
(d)
Answer
(b) X would most likely be in the same group of the Periodic Table as magnesium (Mg).
Question 16: Which element has
(a) two shells, both of which are completely filled with electrons?
(b) the electronic configuration 2, 8, 2 ?
(c) a total of three shells, with four electrons in its valence shell?
(d) a total of two shells, with three electrons in its valence shell?
(e) twice as many electrons in its second shell as in its first shell?
Answer
(a) Neon has two shells, both of which are completely filled with electrons (2 electrons in
(b) Magnesium has the electronic configuration 2, 8, 2.
(c) Silicon has a total of three shells, with four electrons in its valence shell (2 electrons in
(d) Boron has a total of two shells, with three electrons in its valence shell (2 electrons in
(e) Carbon has twice as many electrons in its second shell as in its first shell (2 electrons in
Question 17:
(a) What property do all elements in the same column of the Periodic Table as boron have in common?
(b) What property do all elements in the same column of the Periodic Table as fluorine have in common?
Answer
(a) All the elements in the same column as boron have the same number of valence electrons
(3). Hence, they all have valency equal to 3.
(b) All the elements in the same column as fluorine have the same number of valence electrons (7). Hence, they all have valency equal to 1.
Question 18: An atom has electronic configuration 2, 8, 7.
(a) What is the atomic number of this element?
(b) To which of the following elements would it be chemically similar? (Atomic numbers are given in parentheses.)
N(7) F(9) P(15)
Answer
(a) The atomic number of this element is 17.
(b) It would be chemically similar to
Question 19: The position of three elements A, B and C in the Periodic Table are shown below Group 16 Group 17

A
B
(a) State whether
(b) State whether
(c) Will C be larger or smaller in size than B?
(d) Which type of ion, cation or anion, will be formed by element A?
Answer
(a) A is a non-metal.
(b) C is less reactive than
(c) C will be smaller in size than B as moving across a period, the nuclear charge increases and therefore, electrons come closer to the nucleus.
(d) A will form an anion as it accepts an electron to complete its octet.
Question 20: Nitrogen (atomic number 7) and phosphorus (atomic number 15) belong to group 15 of the Periodic Table. Write the electronic configuration of these two elements. Which of these will be more electronegative? Why?
Element K L M
Nitrogen 25
Phosphorus2 85
Answer
Nitrogen is more electronegative than phosphorus. On moving down a group, the number of shell increases. Therefore, the valence electrons move away from the nucleus and the effective nuclear charge decreases. This causes the decrease in the tendency to attract electron and hence electronegativity decreases.
Question 21: How does the electronic configuration of an atom relate to its position in the Modern Periodic Table?
In the modern periodic table, atoms with similar electronic configurations are placed in the same column. In a group, the number of valence electrons remains the same. Elements across a period show an increase in the number of valence electrons.
Question 22: In the Modern Periodic Table, calcium (atomic number 20) is surrounded by elements with atomic numbers
The element with atomic number 12 has same chemical properties as that of calcium. This is because both of them have same number of valence electrons (2).
Question 23: Compare and contrast the arrangement of elements in Mendeleev’s periodic Table and the Modern Periodic Table.
not be explained.
Mendeleev’s periodic table
Elements are arranged in thĐownloaded from : http://www.cbseportal.com
1.increasing order of their atomic masses.
There are a total of 7 groups 2. (columns) and 6 periods (rows).
Elements having similar
3.properties were placed directly under one another.
The position of hydrogen could
No distinguishing positions for
- metals and non-metals.
Metals are present at the left
hand side of the periodic table
- whereas non-metals are
present at the right hand side. Elements are arranged in the increasing order of their atomic 1 . numbers.
There are a total of 18 groups (columns) and 7 periods
(rows).
Elements having the same valence shell are present in the 3.same period while elements having the same number of valence electrons are present in the same group.
4.Hydrogen is placed above alkali metals.
Life Processes
Question 1: Why is diffusion insufficient to meet the oxygen requirements of multi-cellular organisms like humans?
Answer
Multicellular organisms such as humans possess complex body designs. They have specialised cells and tissues for performing various necessary functions of the body such as intake of food and oxygen. Unlike unicellular organisms, multicellular cells are not in direct contact with the outside environment. Therefore, diffusion cannot meet their oxygen requirements.
Question 2: What criteria do we use to decide whether something is alive?
Answer
Any visible movement such as walking, breathing, or growing is generally used to decide whether something is alive or not. However, a living organism can also have movements, which are not visible to the naked eye. Therefore, the presence of life processes is a fundamental criterion that can be used to decide whether something is alive or not.
Question 3: What are outside raw materials used for by an organism?
Answer
An organism uses outside raw materials mostly in the form of food and oxygen. The raw materials required by an organism can be quite varied depending on the complexity of the organism and its environment.
Question 4: What processes would you consider essential for maintaining life?
Answer
Life processes such as nutrition, respiration, transportation, excretion, etc. are essential for maintaining life.
Question 5: What are the differences between autotrophic nutrition and heterotrophic nutrition?
Answer
Autotrophic nutrition
Heterotrophic nutrition
(i) Food is synthesised from simple inorganic raw materials such as CO2and water.
(i) Food is obtained directly or indirectly from autotrophs. This food is broken down with the help of enzymes.
(ii) Presence of green pigment (ii) No pigment is required in this type of nutrition. (chlorophyll) is necessary.
(iii)Food is generally prepared during day (iii)Food can be prepared at all times. time.
(iv)All green plants and some bacteria have(iv)All animals and fungi have this type of nutrition. this type of nutrition.
Question 6: Where do plants get each of the raw materials required for photosynthesis?
Answer
The following raw materials are required for photosynthesis:
- The raw material CO2 enters from the atmosphere through stomata.
- Water is absorbed from the soil by the plant roots.
- Sunlight, an important component to manufacture food, is absorbed by the chlorophyll and other green parts of the plants.
Question 7: What is the role of the acid in our stomach?
Answer
The hydrochloric acid present in our stomach dissolves bits of food and creates an acidic medium. In this acidic medium, enzyme pepsinogen is converted to pepsin, which is a proteindigesting enzyme.
Question 8: What is the function of digestive enzymes?
Answer
Digestive enzymes such as amylase, lipase, pepsin, trypsin, etc. help in the breaking down of complex food particles into simple ones. These simple particles can be easily absorbed by the blood and thus transported to all the cells of the body.
Question 9: How is the small intestine designed to absorb digested food?
Answer
The small intestine has millions of tiny finger-like projections called villi. These villi increase the surface area for more efficient food absorption. Within these villi, many blood vessels are present that absorb the digested food and carry it to the blood stream. From the blood stream, the absorbed food is delivered to each and every cell of the body.

Enlarged view of a villus
Question 10: What advantage over an aquatic organism does a terrestrial organism have with regard to obtaining oxygen for respiration?
Answer
Terrestrial organisms take up oxygen from the atmosphere whereas aquatic animals need to utilize oxygen present in the water. Air contains more
Question 11: What are the different ways in which glucose is oxidized to provide energy in various organisms?
Answer
Glucose is first broken down in the cell cytoplasm into a three carbon molecule called pyruvate. Pyruvate is further broken down by different ways to provide energy. The breakdown of glucose by different pathways can be illustrated as follows.

In yeast and human muscle cells, the breakdown of pyruvate occurs in the absence of oxygen whereas in mitochondria, the breakdown of pyruvate occurs in the presence of oxygen.
Question 12: How is oxygen and carbon dioxide transported in human beings?
Answer
Haemoglobin transports oxygen molecule to all the body cells for cellular respiration. The haemoglobin pigment present in the blood gets attached to four
Since haemoglobin pigment has less affinity for

Transportation of
Question 13: How are the lungs designed in human beings to maximize the area for exchange of gases?
Answer
The exchange of gases takes place between the blood of the capillaries that surround the alveoli and the gases present in the alveoli. Thus, alveoli are the site for exchange of gases. The lungs get filled up with air during the process of inhalation as ribs are lifted up and diaphragm is flattened. The air that is rushed inside the lungs fills the numerous alveoli present in the lungs.
Each lung contains 300-350 million alveoli. These numerous alveoli increase the surface area for gaseous exchange making the process of respiration more efficient.
Question 14: What are the components of the transport system in human beings? What are the functions of these components?
Answer
The main components of the transport system in human beings are the heart, blood, and blood vessels.
-Heart pumps oxygenated blood throughout the body. It receives deoxygenated blood from the various body parts and sends this impure blood to the lungs for oxygenation.
-The blood vessels (arteries, veins, and capillaries) carry blood either away from the heart to various organs or from various organs back to the heart.
Question 15: Why is it necessary to separate oxygenated and deoxygenated blood in mammals and birds?
Answer
Warm-blooded animals such as birds and mammals maintain a constant body temperature by cooling themselves when they are in a hotter environment and by warming their bodies when they are in a cooler environment. Hence, these animals require more oxygen (O2) for more cellular respiration so that they can produce more energy to maintain their body temperature.
Thus, it is necessary for them to separate oxygenated and de-oxygenated blood, so that their circulatory system is more efficient and can maintain their constant body temperature.
Question 16: What are the components of the transport system in highly organised plants?
Answer
In highly organised plants, there are two different types of conducting tissues - xylem and phloem. Xylem conducts water and minerals obtained from the soil (via roots) to the rest of the plant. Phloem transports food materials from the leaves to different parts of the plant body.
Question 17: How are water and minerals transported in plants?
Answer
The components of xylem tissue (tracheids and vessels) of roots, stems, and leaves are interconnected to form a continuous system of water-conducting channels that reaches all parts of the plant. Transpiration creates a suction pressure, as a result of which water is forced into the xylem cells of the roots. Then there is a steady movement of water from the root xylem to all the plant parts through the interconnected water-conducting channels.

Components of xylem tissue
Question 18: How is food transported in plants?
Phloem transports food materials from the leaves to different parts of the plant body. The transportation of food in phloem is achieved by utilizing energy from ATP. As a result of this, the osmotic pressure in the tissue increases causing water to move into it. This pressure moves the material in the phloem to the tissues which have less pressure. This is helpful in moving materials according to the needs of the plant. For example, the food material, such as sucrose, is transported into the phloem tissue using ATP energy.

Components of phloem tissue
Question 19: Describe the structure and functioning of nephrons.
Answer
Nephrons are the basic filtering units of kidneys. Each kidney possesses large number of nephrons, approximately 1-1.5 million. The main components of the nephron are glomerulus, Bowman’s capsule, and a long renal tubule.

Structure of a nephron
Functioning of a nephron:
- The blood enters the kidney through the renal artery, which branches into many capillaries associated with glomerulus.
- The water and solute are transferred to the nephron at Bowman’s capsule. In the
- proximal tubule, some substances such as amino acids, glucose, and salts are selectively reabsorbed and unwanted molecules are added in the urine.
- The filtrate then moves down into the loop of Henle, where more water is absorbed.
- From here, the filtrate moves upwards into the distal tubule and finally to the collecting duct.
- Collecting duct collects urine from many nephrons.
The urine formed in each kidney enters a long tube called ureter. From ureter, it gets transported to the urinary bladder and then into the urethra.
Question 20: What are the methods used by plants to get rid of excretory products?
Answer
Plants can get rid of excess of water by transpiration. Waste materials may be stored in the cell vacuoles or as gum and resin, especially in old xylem. It is also stored in the leaves that later fall off.
Question 21: How is the amount of urine produced regulated?
Answer
The amount of urine produced depends on the amount of excess water and dissolved wastes present in the body. Some other factors such as habitat of an organism and hormone such as Anti-diuretic hormone (ADH) also regulates the amount of urine produced.
Question 22: The kidneys in human beings are a part of the system for
(a) nutrition.
(b) respiration.
(c) excretion.
(d) transportation.
(c) In human beings, the kidneys are a part of the system for excretion.
Question 23: The xylem in plants are responsible for
(a) transport of water.
(b) transport of food.
(c) transport of amino acids.
(d) transport of oxygen.
(a) In a plant, the xylem is responsible for transport of water.
Question 24: The autotrophic mode of nutrition requires
(a) carbon dioxide and water.
(b) chlorophyll.
(c) sunlight.
(d) all of the above.
Answer
(d) The autotrophic mode of nutrition requires carbon dioxide, water, chlorophyll and sunlight.
Question 25: The breakdown of pyruvate to give carbon dioxide, water and energy takes place in
(a) cytoplasm.
(b) mitochondria.
(c) chloroplast.
(d) nucleus.
Answer
(b) The breakdown of pyruvate to give carbon dioxide, water and energy takes place in mitochondria.
Question 26: How are fats digested in our bodies? Where does this process take place?
Answer
Fats are present in the form of large globules in the small intestine. The small intestine gets the secretions in the form of bile juice and pancreatic juice respectively from the liver and the pancreas. The bile salts (from the liver) break down the large fat globules into smaller globules so that the pancreatic enzymes can easily act on them. This is referred to as emulsification of fats. It takes place in the small intestine.
Question 27: What are the necessary conditions for autotrophic nutrition and what are its byproducts?
Answer
Autotrophic nutrition takes place through the process of photosynthesis. Carbon dioxide, water, chlorophyll pigment, and sunlight are the necessary conditions required for autotrophic nutrition. Carbohydrates (food) and
Question 28: What are the differences between aerobic and anaerobic respiration? Name some organisms that use the anaerobic mode of respiration.
Answer
Aerobic respiration
1.It occurs in the presence of
Anaerobic respiration
1.It occurs in the absence of
2.It involves the exchange of gases between the organism and the2.Exchange of gases is outside environment. absent.
3.It occurs in cytoplasm and mitochondria.
4.It always releases
5.It yields 36
ATPs.
3.It occurs only in cytoplasm.
4.End products vary.
5.It yields only 2
ATPs.
Anaerobic respiration occurs in the roots of some waterlogged plants, some parasitic worms, animal muscles, and some micro-organisms such as yeasts.
Question 29: How are the alveoli designed to maximise the exchange of gases?
Answer
The alveoli are the small balloon-like structures present in the lungs. The walls of the alveoli consist of extensive network of blood vessels. Each lung contains 300-350 million alveoli, making it a total of approximately 700 million in both the lungs. The alveolar surface when spread out covers about

Alveoli and capillaries
Question 30: What would be the consequences of a deficiency of haemoglobin in our bodies?
Answer
Haemoglobin is the respiratory pigment that transports oxygen to the body cells for cellular respiration. Therefore, deficiency of haemoglobin in blood can affect the oxygen supplying capacity of blood. This can lead to deficiency of oxygen in the body cells. It can also lead to a disease called anaemia.
Question 31: Describe double circulation in human beings. Why is it necessary?
Answer
The human heart is divided into four chambers - the right atrium, the right ventricle, the left atrium, and the left ventricle.
Flow of blood in the heart:
-The heart has superior and inferior vena cava, which carries de-oxygenated blood from the upper and lower regions of the body respectively and supplies this de-oxygenated blood to the right atrium of the heart.

Flow of blood in the human heart
-The right atrium then contracts and passes the de-oxygenated blood to the right ventricle, through an auriculo-ventricular aperture.
-Then the right ventricle contracts and passes the de-oxygenated blood into the two pulmonary arteries, which pumps it to the lungs where the blood becomes oxygenated. From the lungs, the pulmonary veins transport the oxygenated blood to the left atrium of the heart. Then the
- left atrium contracts and through the auriculo-ventricular aperture, the oxygenated blood enters the left ventricle.
- The blood passes to aorta from the left ventricle. The aorta gives rise to many arteries that distribute the oxygenated blood to all the regions of the body.

Schematic diagram of blood circulation in humans
Therefore, the blood goes twice through the heart. This is known as double circulation.
Importance of double circulation:
The separation of oxygenated and de-oxygenated blood allows a more efficient supply of oxygen to the body cells. This efficient system of oxygen supply is very useful in warm-blooded animals such as human beings.
As we know, warm-blooded animals have to maintain a constant body temperature by cooling themselves when they are in a hotter environment and by warming their bodies when they are in a cooler environment. Hence, they require more O2 for more respiration so that they can produce more energy to maintain their body temperature. Thus, the circulatory system of humans is more efficient because of the double circulatory heart.
Question 32: What are the differences between the transport of materials in xylem and phloem?
Answer
Transport of materials in xylem Transport of materials in phloem
(i) Xylem tissue helps in the transport of water and minerals.
(i) Phloem tissue helps in the transport of food.
(ii)Water is transported upwards from roots to all other plant parts.
(ii)Food is transported in both upward and downward directions.
(iii)Transport in xylem occurs with the help of simple physical forces such as transpiration pull.
(iii)Transport of food in phloem requires energy in the form of ATP.
Question 13: Compare the functioning of alveoli in the lungs and nephrons in the kidneys with respect to their structure and functioning.
Answer
Alveoli Nephron
Structure Structure
(i) Alveoli are tiny balloon-like (i) Nephrons are tubular structures present inside the kidneys. structures present inside the lungs.
(ii) Nephrons are made of glomerulus, bowman’s capsule, and
(ii) The walls of the alveoli are one a long renal tube. It also contains a cluster of thin-walled cell thick and it contains an capillaries.
extensive network of blood capillaries.

Function
(i) The exchange of
alveoli. and solute are transferred to the nephron at Bowman’s

(ii) Alveoli are the site of gaseous exchange.

Function capsule. Then the filtrate moves through the proximal tubule and then down into the loop of henle. From henle’s loop, filtrate passes into the distal tubule and then to the collecting duct. The collecting duct collects the urine from many nephrons and passes it to the ureter. During the flow of filtrate, some substances such as glucose, amino acids, and water are selectively re-absorbed.

(ii) Nephrons are the basic filtr
NCERT Solutions for Class 10th: Ch 7 Control and Coordination Science
In Text QuestionsPage No: 119
- What is the difference between a reflex action and walking?
Answer
A reflex action is voluntary action which is a rapid and automatic response to stimuli while walking is a voluntary action which requires our thinking and in our control.
- What happens at the synapse between two neurons?
Answer
A synapse is the gap between the two neurons. At synapse the electrical signals converted into chemicals that can easily cross over the gap and pass on to the next neurons where it again converted into electrical signals.
- Which part of the brain maintains posture and equilibrium of the body?
Cerebellum
- How do we detect the smell of an agarbatti (incense stick)?
Answer
When the smell of the incense stick reaches to our nose then the olfactory receptors present in our nose detects it send this information in fore brain in the form of electrical signals. Fore brain interprets this information as the smell of incense stick where it is already stored.
- What is the role of the brain in reflex action?
Answer
Brain has no direct involvement in reflex action. It is mainly controlled by Spinal Cord as these action not requires thinking and are very quick action.
Page No: 122
- What are plant hormones?
Answer
Plant hormones are the fluids which are secreted within the plant also known as phytohormones. Plant hormones regulate the growth and development of the plant. Examples of plant hormones are auxin, gibberellins etc.
- How is the movement of leaves of the sensitive plant different from the movement of a shoot towards light?
Answer
The movements of the leaves of the sensitive plant are touch sensitive and independent of growth while the movement of the shoot towards light is growth related and known as phototropism.
- Give an example of a plant hormone that promotes growth.
Auxin
- How do auxins promote the growth of a tendril around a support?
Answer
When tendrils come in contact with any support, the part of the tendril in contact with the object does not grow as rapidly as the part of the tendril away from the object. This is caused by the action of auxin hormone. Less auxin occurs on the side of contact as compared to the free side as a result, auxin promotes growth on the free side and the tendrils coil around the support.
- Design an experiment to demonstrate hydrotropism.
Answer
Take two small beakers and label them as A and B. Fill beaker A with water. Now make a cylindrical-shaped roll from a filter paper and keep it as a bridge between beaker
Observation:
The roots of the germinating seeds will grow towards beaker A.

This experiment demonstrates the phenomenon of hydrotropism.
Page No: 125
1. How does chemical coordination take place in animals?
Answer
Chemical coordination takes place in animals with the help of hormones. Hormones are the chemical fluids that are secreted by the glands of the endocrine system. Hormones regulate the overall growth and development of the animals.
2. Why is the use of iodised salt advisable?
Answer
lodine stimulates the thyroid gland to produce thyroxin hormone. It regulates carbohydrate, fat, and protein metabolism in our body. Deficiency of this hormone results in the enlargement of the thyroid gland. This can lead to goitre, a disease characterized by swollen neck. Therefore, iodised salt is advised for normal functioning of the thyroid gland.
- How does our body respond when adrenaline is secreted into the blood?
Answer
When someone is in danger or in emergency then adrenal gland secrete adrenaline hormone. It is secreted directly into the blood and is transported to different parts of the body. It speeds up the heartbeat and hence supplies more oxygen to the muscles. This results in increasing breathing rate and blood pressure which enable them to fight with such urgent situation.
- Why are some patients of diabetes treated by giving injections of insulin?
Answer
Diabetes is caused due to less or no secretion of hormone insulin by pancreas. In such a person, blood sugar level is high. Insulin converts extra sugar present in blood into glycogen. Thus, patients suffering from diabetes are given insulin injection to control their blood sugar level.
Excercise
- Which of the following is a plant hormone?
(a) Insulin
(b) Thyroxin
(c) Oestrogen
(d) Cytokinin
(d) Cytokinin
- The gap between two neurons is called a
(a) dendrite.
(b) synapse.
(c) axon.
(d) impulse.
(b) synapse.
Page No: 126
- The brain is responsible for
(a) thinking.
(b) regulating the heart beat.
(c) balancing the body.
(d) all of the above.
(d) all of the above.
- What is the function of receptors in our body? Think of situations where receptors do not work properly. What problems are likely to arise?
Answer
Functions of receptors:
When the receptors are damaged, the external stimuli transferring signals to the brain are not felt. For example, in the case of damaged receptors, if we accidentally touch any hot object, then our hands might get burnt as damaged receptors cannot perceive the external stimuli of heat and pain.
- Draw the structure of a neuron and explain its function.
Answer

Functions of the three parts of a neuron:
6. How does phototropism occur in plants?
Answer
The growth movement in plants in response to light stimulus is known as phototropism. The shoots show positive phototropism and the roots show negative phototropism. This means that the shoots bend towards the source of light whereas the roots bend away from the light source. For Example: The flower head of sunflower is positively phototropic and hence it moves from east to west along with the sun.
7. Which signals will get disrupted in case of a spinal cord injury?
Answer
In case of the spinal cord injury, the signals coming from the nerves as well as the signals coming to the receptors will be disrupted. As both these signals meet in a bundle in spinal cord so there is any spinal cord injury then both these signals are disrupted.
8. How does chemical coordination occur in plants?
Answer
Chemical coordination occurs in plants with the help of plant hormones. Different plant hormones help to coordinate growth, development, and responses to the environment. They are synthesized at places away from where they act and diffuse to the area for action, For example, auxin promotes cell growth, gibberellins promote stem growth, cytokinins promote cell division and abscisic acid inhibits growth and its effects include wilting of leaves.
- What is the need for a system of control and coordination in an organism?
Answer
There are various organs in an organism. These organs must be carefully controlled and coordinated for the survival of an organisms. In the body of an organism various fluids are secreted from the glands of the endocrine system. These hormones are responsible for the overall growth and development of an organism. All others daily decision that includes voluntary and involuntary action are controlled by central nervous system(CNS).
- How are involuntary actions and reflex actions different from each other?
Answer
Involuntary action is the set of muscle movement which do not require thinking. But it is controlled by brain for example beating of heart beat while on the other hand, the reflex action is rapid and spontaneous action in response to any stimulus. For example closing of eyes immediately when bright light is focused.
11. Compare and contrast nervous and hormonal mechanisms for control and coordination in animals.
Answer
| Nervous System Mechanism | Hormonal System Mechanism |
|---|---|
| It is consist of nerve impulses between PNS, CNS and Brain. |
It consists of endocrine system which secretes hormones directly into blood. |
| The axons and dendrites transmit the information through a coordinated effort. |
The information is transmitted or transported through blood. |
| The flow of information is rapid and the response is quick. |
The information travels slowly and the response is slow. |
| Nerve impulses are not specific in their action. |
Each hormone has specific actions. |
|---|---|
| Effects are short lived. | It has prolonged effects. |
- What is the difference between the manner in which movement takes place in a sensitive plant and the movement in our legs?
Answer
| Movement in sensitive plants | Movement in our legs |
|---|---|
| The movement in a sensitive plant is a response to stimulus(touch) which is a involuntary action. |
Movement in our legs is a voluntary action. |
| No special tissue is there for the transfer of information |
A complete system CNS and PNS is there for the information exchange. |
| Plant cells do not have specialised protein for movements. |
Animal cells have specialised protein which help muscles to contract. |
Heredity and Evolution
Question 1: If a trait A exists in 10% of a population of an asexually reproducing species and a trait B exists in
Answer
In asexual reproduction, the reproducing cells produce a copy of their DNA through some chemical reactions. However, this copying of DNA is not accurate and therefore, the newly formed DNA has some variations.

It can be easily observed in the above figure that in asexual reproduction, very few variations are allowed. Therefore, if a trait is present in only
Question 2: How does the creation of variations in a species promote survival?
Answer
Sometimes for a species, the environmental conditions change so drastically that their survival becomes difficult. For example, if the temperature of water increases suddenly, most of the bacteria living in that water would die. Only few variants resistant to heat would be able to survive. If these variants were not there, then the entire species of bacteria would have been destroyed. Thus, these variants help in the survival of the species.
However, not all variations are useful. Therefore, these are not necessarily beneficial for the individual organisms.
Question 3: How do Mendel’s experiments show that traits may be dominant or recessive?
Answer
Mendel selected true breeding tall (TT) and dwarf (tt) pea plants. Then, he crossed these two plants. The seeds formed after fertilization were grown and these plants that were formed represent the first filial or F1 generation. All the F1 plants obtained were tall.

Progeny
( 
Tall plant
Tall plant

Cross-pollination of tall and short plant
Then, Mendel self-pollinated the F1 plants and observed that all plants obtained in the F2 generation were not tall. Instead, one-fourth of the F2 plants were short.

Self-pollination of F1 plants
From this experiment, Mendel concluded that the F1 tall plants were not true breeding. They were carrying traits of both short height and tall height. They appeared tall only because the tall trait is dominant over the dwarf trait.
Question 4: How do Mendel’s experiments show that traits are inheritedindependently?
Answer
Mendel crossed pea plants having round green seeds (RRyy) with pea plants having wrinkled yellow seeds (rrYY).

An example of dihybrid crosses
Since the F1 plants are formed after crossing pea plants having green round seeds and pea plants having yellow wrinkled seeds, F1 generation will have both these characters in them. However, as we know that yellow seed colour and round seeds are dominant characters, therefore, the F1 plants will have yellow round seeds.
Then this F1 progeny was self-pollinated and the F2 progeny was found to have yellow round seeds, green round seeds, yellow wrinkled seeds, and green wrinkled seeds in the ratio of 9:3:3:1.

Independent inheritance of two different traits
In the above cross, more than two factors are involved, and these are independently inherited.
Question 4: A man with blood group A marries a woman with blood group
Answer
No. This information is not sufficient to determine which of the traits - blood group A or
Blood group A can be genotypically AA or AO. Hence, the information is incomplete to draw any such conclusion.
Question 5: How is the sex of the child determined in human beings?
Answer
In human beings, the females have two
The gametes, as we know, receive half of the chromosomes. The male gametes have 22 autosomes and either X or Y sex chromosome.
Type of male gametes: 22+X OR 22+ Y.
However, since the females have XX sex chromosomes, their gametes can only have
Type of female gamete: 22+ X

Sex determination in humans
Thus, the mother provides only
Question 6: What are the different ways in which individuals with a particular trait may increase in a population?
Answer
Individuals with a particular trait may increase in a population as a result of the following:
(i) Natural selection: When that trait offers some survival advantage.
(ii) Genetic drift: When some genes governing that trait become common in a population.
(iii) When that trait gets acquired during the individual’s lifetime.
Question 7: Why are traits acquired during the life-time of an individual not inherited?
Answer
This happens because an acquired trait involves change in non-reproductive tissues (somatic cells) which cannot be passed on to germ cells or the progeny. Therefore, these traits cannot be inherited.
Question 8: Why are the small numbers of surviving tigers a cause of worry from the point of view of genetics?
Small numbers of tigers means that fewer variations in terms of genes are available. This means that when these tigers reproduce, there are less chances of producing progeny with some useful variations. Hence, it is a cause of worry from the point of view of genetics.
Question 9: What factors could lead to the rise of a new species?
Answer
Natural selection, genetic drift and acquisition of traits during the life time of an individual can give rise to new species. ill geographical isolation be a major factor in the speciation of a self-pollinating plant species? Why or why not? Geographical isolation can prevent the transfer of pollens among different plants. However, since the plants are self-pollinating, which means that the pollens are transferred from the anther of one flower to the stigma of the same flower or of another flower of the same plant, geographical isolation cannot prevent speciation in this case.
Question 10: Will geographical isolation be a major factor in the speciation of an organism that reproduces asexually? Why or why not?
Answer
Geographical isolation prevents gene flow between populations of a species whereas asexual reproduction generally involves only one individual. In an asexually reproducing organism, variations can occur only when the copying of DNA is not accurate. Therefore, geographical isolation cannot prevent the formation of new species in an asexually reproducing organism.
Question 11: Give an example of characteristics being used to determine how close two species are in evolutionary terms.
Answer
The presence of feathers in dinosaurs and birds indicates that they are evolutionarily related. Dinosaurs had feathers not for flying but instead these feathers provided insulation to these warm-blooded animals. However, the feathers in birds are used for flight. This proves that reptiles and birds are closely related and that the evolution of wings started in reptiles.
Question 12: Can the wing of a butterfly and the wing of a bat be considered homologous organs? Why or why not?
Answer
The wing of a butterfly and the wing of a bat are similar in function. They help the butterfly and the bat in flying. Since they perform similar function, they are analogous organs and not homologous.
Question 13: What are fossils? What do they tell us about the process of evolution?
Answer
Fossils are the remains of organisms that once existed on earth. They represent the ancestors of plants and animals that are alive today. They provide evidences of evolution by revealing the characteristics of the past organism and the changes that have occurred in these organisms to give rise to the present organisms.
Question 14: Why are human beings who look so different from each other in terms of size, colour and looks said to belong to the same species?
Answer
A species is a group of organisms that are capable of interbreeding to produce a fertile offspring. Skin colour, looks, and size are all variety of features present in human beings. These features are generally environmentally controlled. Various human races are formed based on these features. However, there is no biological basis to this concept of races. Therefore, all human beings are a single species as humans of different colour, size, and looks are capable of reproduction and can produce a fertile offspring.
Question 15: In evolutionary terms, can we say which among bacteria, spiders, fish and chimpanzees have a ‘better’ body design? Why or why not?
Answer
Evolution cannot always be equated with progress or better body designs. Evolution simply creates more complex body designs. However, this does not mean that the simple body designs are inefficient. In fact, bacteria having a simple body design are still the most cosmopolitan organisms found on earth. They can survive hot springs, deep sea, and even freezing environment.
Therefore, bacteria, spiders, fish, and chimpanzees are all different branches of evolution.
Question 16: A Mendelian experiment consisted of breeding tall pea plants bearing violet flowers with short pea plants bearing white flowers. The progeny all bore violet flowers, but almost half of them were short. This suggests that the genetic make-up of the tall parent can be depicted as
(a) TTWW
(b) TTww
(c) TtWW
(d)
Answer
(c) The genetic make-up of the tall parent can be depicted as TtWW
Since all the progeny bore violet flowers, it means that the tall plant having violet flowers has WW genotype for violet flower colour.
Since the progeny is both tall and short, the parent plant was not a pure tall plant. Its genotype must be Tt.
Therefore, the cross involved in the given question is
Therefore, half the progeny is tall, but all of them have violet flowers.
Question 17: An example of homologous organs is
(a) our arm and a dog’s fore-leg.
(b) our teeth and an elephant’s tusks.
(c) potato and runners of grass.
(d) all of the above.
Answer
(b)An example of homologous organs is our teeth and an elephant’s tusks.
Question 18: In evolutionary terms, we have more in common with
(a) a Chinese school-boy.
(b) a chimpanzee.
(c) a spider.
(d) a bacterium.
Answer
(a) In evolutionary terms, we have more in common with a Chinese school boy.
Question 19: A study found that children with light-coloured eyes are likely to have parents with light-coloured eyes. On this basis, can we say anything about whether the light eye colour trait is dominant or recessive? Why or why not?
Answer
Let us assume that children with light-coloured eyes can either have LL or Ll or ll genotype. If the children have LL genotype, then their parents will also be of LL genotype.

LL
If the children with light-coloured eyes have
ll
Therefore, it cannot be concluded whether light eye colour is dominant or recessive.
Question 20: How are the areas of study - evolution and classification - interlinked?
Answer
Classification involves grouping of organism into a formal system based on similarities in internal and external structure or evolutionary history.
Two species are more closely related if they have more characteristics in common. And if two species are more closely related, then it means they have a more recent ancestor.
For example, in a family, a brother and sister are closely related and they have a recent common ancestor i.e., their parents. A brother and his cousin are also related but less than the sister and her brother. This is because the brother and his cousin have a common ancestor i.e., their grandparents in the second generation whereas the parents were from the first generation.
With subsequent generations, the variations make organisms more different than their ancestors.
This discussion clearly proves that we classify organisms according to their resemblance which is similar to creating an evolutionary tree.
Question 21: Explain the terms analogous and homologous organs with examples.
Answer
Homologous organs are similar in origin (or are embryologically similar) but perform different functions. For example, the forelimbs of humans and the wings of birds look different externally but their skeletal structure is similar. It means that their origin is similar (as wings in birds are modifications of forearm) but functions are different - the wings help in flight whereas human forearm helps in various activities.

Homologous organs
Analogous organs, on the other hand, have different origin but perform similar functions. For example, the wings of a bird and a bat are similar in function but this similarity does not mean that these animals are more closely related. If we carefully look at these structures, then we will find that the wings of a bat are just the folds of skin that are stretched between its fingers whereas the wings of birds are present all along the arm. Therefore, these organs are analogous organs.
Question 22: Outline a project which aims to find the dominant coat colour in dogs.
Answer
Dogs have a variety of genes that govern coat colour. There are at least eleven identified gene series (A, B, C, D, E, F, G, M, P, S, T) that influence coat colour in dog.
A dog inherits one gene from each of its parents. The dominant gene gets expressed in the phenotype. For example, in the B series, a dog can be genetically black or brown.
Let us assume that one parent is homozygous black (BB), while the other parent is homozygous brown (bb)
bb BB
B B
In this case, all the offsprings will be heterozygous (Bb).
Since black (B) is dominant, all the offsprings will be black. However, they will have both B and
If such heterozygous pups are crossed, they will produce 25% homozygous black (BB),
B b
Question 23: Explain the importance of fossils in deciding evolutionary relationships.
Answer
Fossils are the remains of the organism that once existed on earth. They represent the ancestors of the plants and animals that are alive today. They provide evidences of evolution by revealing the characteristics of the past organisms and the changes that have occurred in these organisms to give rise to the present organisms. Let us explain the importance of fossils in deciding evolutionary history with the help of the following example.
Around 100 million years ago, some invertebrates died and were buried in the soil in that area. More sediment accumulated on top of it turning it into sedimentary rock.
At the same place, millions of years later, some dinosaurs died and their bodies were buried on top of the sedimentary rock. The mud containing dinosaurs also turned into a rock.
Then, millions of years later, some horse-like creatures died in that area and got fossilized in rocks above the dinosaur fossils.
Some time later, due to soil erosion or floods in that area, the rocks containing horse-like fossils are exposed.
If that area is excavated deeper, then the dinosaur and invertebrates fossils can also be found. Thus, by digging that area, scientists can easily predict that horse-like animals evolved later than the dinosaurs and the invertebrates.
Thus, the above example suggests that the fossils found closer to the surface of the earth are more recent ones than the fossils present in deeper layers.

Layers of fossils
Question 24: What evidence do we have for the origin of life from inanimate matter?
Answer
A British scientist, J.B.S. Haldane, suggested that life originated from simple inorganic molecules. He believed that when the earth was formed, it was a hot gaseous mass containing elements such as nitrogen, oxygen, carbon, hydrogen, etc. These elements combined to form molecules like water (H2O), carbon dioxide (CO2), methane (CH4), ammonia (NH3), etc.
After the formation of water, slowly the earth surface cooled and the inorganic molecules interacted with one another in water to form simple organic molecules such as sugars, fatty acids, amino acids, etc. The energy for these reactions was provided by solar radiations, lightning, volcanic eruptions, etc.
This was proved by the experiment of Stanley L. Miller and Harold C. Urey in 1953.
They took a mixture of water (H2O), methane (CH4), ammonia (NH3), and hydrogen gas (H2) in a chamber and sparks were passed through this mixture using two electrodes. After one week, 15

Miller and Urey experiment
Question 25: Explain how sexual reproduction gives rise to more viable variations than asexual reproduction. How does this affect the evolution of those organisms that reproduce sexually?
Answer
In sexual reproduction, two individuals having different variations combine their DNA to give rise to a new individual. Therefore, sexual reproduction allows more variations, whereas in asexual reproduction, chance variations can only occur when the copying of DNA is not accurate.
Additionally, asexual reproduction allows very less variations because if there are more variations, then the resultant DNA will not be able to survive inside the inherited cellular apparatus.
However, in sexual reproduction, more variations are allowed and the resultant DNA is also able to survive, thus making the variations viable.
Variation and Evolution: Variants help the species to survive in all the conditions. Environmental conditions such as heat, light, pests, and food availability can change suddenly at only one place. At that time, only those variants resistant to these conditions would be able to survive. This will slowly lead to the evolution of a better adapted species. Thus, variation helps in the evolution of sexually reproducing organisms.
Question 26: How is the equal genetic contribution of male and female parents ensured in the progeny?
Answer
In human beings, every somatic cell of the body contains 23 pairs of chromosomes. Out of these 23 pairs, the first 22 pairs are known as autosomes and the remaining one pair is known as sex chromosomes represented as
Females have two X chromosomes and males have one X and one Y chromosome.
The gamete receives half of the chromosomes. Therefore, the male gametes have 22 autosomes and either
The female gamete, on the other hand, has 22 autosomes and X chromosome.
During reproduction, the male and female gametes fuse and thus the progeny receives 22 autosomes and one

Father
22 pairs of autosomes
XY


Mother
22 pairs of autosomes

Gamete

22 pairs of autosomes OR 22 pairs of autosomes
XY
Question 27: Only variations that confer an advantage to an individual organism will survive in a population. Do you agree with this statement? Why or why not?
Answer
In species, variations that offer survival advantages are naturally selected. Individuals adjust to their environments with the help of these selected variations and consequently these variations are passed on to their progeny. Evolution of organisms occurs as a result of this natural selection.
However, there can be some other variations, which do not offer any survival advantage and arise only accidentally. Such variations in small populations can change the frequency of some genes even if they are not important for survival.
This accidental change in the frequency of genes in small populations is referred to as genetic drift.
Thus, genetic drift provides diversity (variations) without any survival advantage.
Organisms Reproduce
Question 1: What is the importance of DNA copying in reproduction?
Answer
DNA (Deoxyribonucleic acid) is the genetic material found in the chromosomes, which are present in the nucleus of a cell. The DNA is the information site for making proteins and each specific type of protein leads to a specific type of body design.
Thus, it is the DNA molecule that determines the body design of an individual. Therefore, it can be concluded that it is the DNA that gets transferred from parents to offsprings and makes them look similar.

DNA determines body structure
Question 2: Why is variation beneficial to the species but not necessarily for the individual?
Answer
Variations are beneficial to the species than individual because sometimes for a species, the environmental conditions change so drastically that their survival becomes difficult. For example, if the temperature of water increases suddenly, then most of the bacteria living in that water would die. Only few variants that are resistant to heat would be able to survive. However, if these variants were not there, then the entire species of bacteria would have been destroyed. Thus, these variants help in the survival of the species. However, all variations are not necessarily beneficial for the individual organisms.
Question 3: How does binary fission differ from multiple fission?
Answer
In binary fission, a single cell divides into two equal halves. Amoeba and Bacteria divide by binary fission.

Binary fission in Amoeba
In multiple fission, a single cell divides into many daughter cells simultaneously. Amoeba andPlasmodium divide by multiple fission.

Multiple fission in Plasmodium
Question 4: How will an organism be benefited if it reproduces through spores?
Answer
There are many advantages, if an organism reproduces through spores.
Advantages of spore formation:
- Large numbers of spores are produced in one sporangium.
- Spores are covered by thick walls to prevent dehydration under unfavourable conditions.
Question 5: Can you think of reasons why more complex organisms cannot give rise to new individuals through regeneration?
Answer
Simple organisms such as Hydra and Planaria are capable of producing new individuals through the process of regeneration. The process of regeneration involves the formation of new organisms from its body parts. Simple organisms can utilize this method of reproduction as their entire body is made of similar kind of cells in which any part of their body can be formed by growth and development.
However, complex organisms have organ-system level of organization. All the organ systems of their body work together as an interconnected unit. They can regenerate their lost body parts such as skin, muscles, blood, etc. However, they cannot give rise to new individuals through regeneration.
Question 6: Why is vegetative propagation practised for growing some types of plants?
Answer
Vegetative propagation is the ability of the plants to reproduce by producing new plants from the vegetative plant parts such as leaf, stem, or roots under appropriate conditions. This method is the only means of reproduction for some seedless plant varieties such as banana, rose, and jasmine. However, this method of reproduction is also used for agricultural purposes in commercial production of some plants such as sugarcane, grapes, roses, etc.
Question 7: Why is DNA copying an essential part of the process of reproduction?
Answer
DNA (Deoxyribonucleic acid) copying is an essential part of reproduction as it passes genetic information from parents to offspring. It determines the body design of an individual. The reproducing cells produce a copy of their DNA through some chemical reactions and result in two
copies of DNA. The copying of DNA always takes place along with the creation of additional cellular structure. This process is then followed by division of a cell to form two cells.
Question 8: How is the process of pollination different from fertilization?
Answer
Pollination is the process of transfer of pollens from anther to stigma. It occurs with the help of certain pollinators such as air, water, birds, or some insects.
Fertilization, on the other hand, is the fusion of the male and female gametes. It occurs inside the ovule and leads to the formation of zygote.
Question 9: What is the role of the seminal vesicles and the prostate gland?
Answer
The secretions from seminal vesicles and prostate glands lubricate the sperms and provide a fluid medium for easy transport of sperms. Their secretion also provides nutrient in the form of fructose, calcium, and some enzymes.
Question 10: What are the changes seen in girls at the time of puberty?
Answer
Secondary sexual characteristics in girls:
- Increase in breast size and darkening of skin of the nipples present at the tips of the breasts.
- Appearance of hair in the genital area.
- Appearance of hair in other areas of skin like underarms, face, hands, and legs.
- Increase in the size of uterus and ovary.
- Beginning of menstrual cycle.
- More secretion of oil from the skin, which results in the appearance of pimples.
Question 11: How does the embryo get nourishment inside the mother’s body?
Answer
The embryo develops inside the mother’s body for about nine months. Inside the uterus, the outer tissue surrounding the embryo develops finger-like projections called villi. These villi are surrounded by uterine tissue and maternal blood. They provide a large surface area for exchange of oxygen and nutrients. Also, there is a special tissue called placenta, which is embedded in the uterine wall. The embryo receives the oxygen and nutrients from the mother’s blood via the placenta. The waste materials produced by the embryo are also removed through the placenta.
Question 12: If a woman is using a copper-T, will it help in protecting her from sexually transmitted diseases?
Answer
No. Using a copper-T will not provide a protection from sexually transmitted diseases, as it does not prevent the entry of semen. It only prevents the implantation of the embryo in the uterus.
Question 13: Asexual reproduction takes place through budding in
(a) amoeba.
(b) yeast.
(c) plasmodium.
(d) leishmania.
Answer
(b) Asexual reproduction takes place through budding in yeast.
Question 14: Which of the following is not a part of the female reproductive system in human beings?
(a) Ovary
(b) Uterus
(c) Vas deferens
(d) Fallopian tube
Answer
(c) Vas deferens is not a part of the female reproductive system in human beings.
Question 15: The anther contains
(a) sepals.
(b) ovules.
(c) carpel.
(d) pollen grains.
Answer
(d) The anther contains pollen grains.
Question 16: What are the advantages of sexual reproduction over asexual reproduction?
Answer
Advantages of sexual reproduction:
- In sexual reproduction, more variations are produced. Thus, it ensures survival of species in a population.
- The new formed individual has characteristics of both the parents.
- Variations are more viable in sexual mode than in asexual one. This is because in asexual reproduction, DNA has to function inside the inherited cellular apparatus.
Question 17: What are the functions performed by the testis in human beings?
Answer
The testes are the male reproductive organs that are located outside the abdominal cavity within a pouch called scrotum.
Functions of testes:
- Produce sperms
- Produce a hormone called testosterone, which brings about secondary sexual characters in boys.
Question 18: Why does menstruation occur?
Answer
Menstruation is a process in which blood and mucous flows out every month through the vagina. This process occurs every month because one egg is released from the ovary every month and at the same time, the uterus (womb) prepares itself to receive the fertilized egg. Thus, the inner lining of the uterus gets thickened and is supplied with blood to nourish the embryo. If the egg does not get fertilised, then the lining of the uterus breaks down slowly and gets released in the form of blood and mucous from the vagina.
Question 19: Draw a labelled diagram of the longitudinal section of a flower.
Answer

Question 20: What are the different methods of contraception?
Answer
The contraceptive methods can be broadly divided into the following types:
-Natural method
-Barrier method
- Oral contraceptives
- Implants and surgical methods
Question 21: How are the modes for reproduction different in unicellular and multicellular organisms?
Answer
In unicellular organisms, reproduction occurs by the division of the entire cell. The modes of reproduction in unicellular organisms can be fission, budding, etc. whereas in multicellular organisms, specialised reproductive organs are present. Therefore, they can reproduce by complex reproductive methods such as vegetative propagation, spore formation, etc. In more complex multicellular organisms such as human beings and plants, the mode of reproduction is sexual reproduction.
Question 22: How does reproduction help in providing stability to populations of species?
Answer
Living organisms reproduce for the continuation of a particular species. It helps in providing stability to the population of species by producing a new individual that resembles the parents. This is the reason why cats give birth to only cats or dogs give birth to only dogs.
Therefore, reproduction provides stability to populations of dogs or cats or any other species.
Question 23: What could be the reasons for adopting contraceptive methods?
Answer
Contraceptive methods are mainly adopted because of the following reasons:
(i) To prevent unwanted pregnancies.
(ii) To control population rise or birth rate.
(iii) To prevent the transfer of sexually transmitted diseases.
Light - Reflection and Refraction
Question 1: Define the principal focus of a concave mirror.
Answer
Light rays that are parallel to the principal axis of a concave mirror converge at a specific point on its principal axis after reflecting from the mirror. This point is known as the principal focus of the concave mirror.
Question 2: The radius of curvature of a spherical mirror is
Answer
Radius of curvature,
Radius of curvature of a spherical mirror
Hence, the focal length of the given spherical mirror is
Question 3: Name the mirror that can give an erect and enlarged image of an object.
Answer
When an object is placed between the pole and the principal focus of a concave mirror, the image formed is virtual, erect, and enlarged.
Question 4: Why do we prefer a convex mirror as a rear-view mirror in vehicles?
Answer
Convex mirrors give a virtual, erect, and diminished image of the objects placed in front of them. They are preferred as a rear-view mirror in vehicles because they give a wider field of view, which allows the driver to see most of the traffic behind him.
Question 5: Find the focal length of a convex mirror whose radius of curvature is
Answer
Radius of curvature,
Radius of curvature
Hence, the focal length of the given convex mirror is
Question 6: A concave mirror produces three times magnified (enlarged) real image of object placed at
Answer
Magnification produced by a spherical mirror is given by the relation,
Let the height of the object, ho
Then, height of the image,
Object distance,
Here, the negative sign indicates that an inverted image is formed at a distance of 30
Question 7: A ray of light travelling in air enters obliquely into water. Does the light ray bend towards the normal or away from the normal? Why?
Answer
The light ray bends towards the normal.
When a ray of light travels from an optically rarer medium to an optically denser medium, it gets bent towards the normal. Since water is optically denser than air, a ray of light travelling from air into the water will bend towards the normal.
Question 8: Light enters from air to glass having refractive index 1.50. What is the speed of light in the glass? The speed of light in vacuum is
Refractive index of a medium nm is given by,
Speed of light in vacuum,
Refractive index of glass,
Speed of light in the glass,
Question 9: Find out, from Table, the medium having highest optical density. Also find the medium with lowest optical density.
Material Refractive index Material medium Refractive
| medium | index | ||
|---|---|---|---|
| Air | 1.0003 | Canada Balsam | 1.53 |
| Ice | 1.31 | - | - |
| Water | 1.33 | Rock salt | 1.54 |
| Alcohol | 1.36 | - | - |
| Kerosene | 1.44 | Carbon disulphide | 1.63 |
| Fused | Dense | ||
| quartz | 1.46 | flint glass | 1.65 |
| Turpentine oil | 1.47 | Ruby | 1.71 |
| Benzene | 1.50 | Sapphire | 1.77 |
| Crown | 2.42 | ||
| glass | 1.52 | Diamond |
Answer
Highest optical density = Diamond
Lowest optical density
Optical density of a medium is directly related with the refractive index of that medium. A medium which has the highest refractive index will have the highest optical density and viceversa.
It can be observed from table 10.3 that diamond and air respectively have the highest and lowest refractive index. Therefore, diamond has the highest optical density and air has the lowest optical density.
Question 10: You are given kerosene, turpentine and water. In which of these does the light travel fastest? Use the information given in Table.
Material Refractive indexMaterial medium Refractive
medium index
Air
| Ice | 1.31 | - | |
|---|---|---|---|
| Water | 1.33 | Rock salt | 1.54 |
| Alcohol | 1.36 | - | - |
| Kerosene | 1.44 | Carbon disulphide | 1.63 |
| Fused | |||
| quartz | 1.46 | Dense | |
| Turpentine oil | 1.47 | Ruby | 1.65 |
| Benzene | 1.50 | Sapphire | 1.71 |
| Crown | |||
| glass | 1.52 | Diamond | 2.42 |
Answer
Speed of light in a medium is given by the relation for refractive index (nm). The relation is given as
It can be inferred from the relation that light will travel the slowest in the material which has the highest refractive index and travel the fastest in the material which has the lowest refractive index.
It can be observed from table 10.3 that the refractive indices of kerosene, turpentine, and water are 1.44, 1.47, and 1.33 respectively. Therefore, light travels the fastest in water.
Question 11: The refractive index of diamond is 2.42. What is the meaning of this statement?
Answer
Refractive index of a medium
Where,
The refractive index of diamond is 2.42 . This suggests that the speed of light in diamond will reduce by a factor 2.42 compared to its speed in air.
Question 12: Define 1 dioptre of power of a lens.
Answer
Power of lens is defined as the reciprocal of its focal length. If
The S.I. unit of power of a lens is Dioptre. It is denoted by D.
1 dioptre is defined as the power of a lens of focal length 1 metre.
Question 13: A convex lens forms a real and inverted image of a needle at a distance of
Answer
When an object is placed at the centre of curvature, 2F1, of a convex lens, its image is formed at the centre of curvature, 2F2, on the other side of the lens. The image formed is inverted and of the same size as the object, as shown in the given figure.

It is given that the image of the needle is formed at a distance of
Hence, the needle is placed in front of the lens at a distance of
Object distance,
Image distance,
Focal length
According to the lens formula,
Power of the lens,
Hence, the power of the given lens is
Question 14: Find the power of a concave lens of focal length
Answer
Focal length of concave lens,
Power of a lens,
Here, negative sign arises due to the divergent nature of concave lens.
Hence, the power of the given concave lens is
Question 15: Which one of the following materials cannot be used to make a lens?
(a) Water
(b) Glass
(c) Plastic
(d) Clay
Answer
(d) A lens allows light to pass through it. Since clay does not show such property, it cannot be used to make a lens.
Question 16: The image formed by a concave mirror is observed to be virtual, erect and larger than the object. Where should be the position of the object?
(a) Between the principal focus and the centre of curvature
(b) At the centre of curvature
(c) Beyond the centre of curvature
(d) Between the pole of the mirror and its principal focus
Question 17: Where should an object be placed in front of a convex lens to get a real image of the size of the object?
(a) At the principal focus of the lens
(b) At twice the focal length
(c) At infinity
(d) Between the optical centre of the lens and its principal focus.
Answer
(b) When an object is placed at the centre of curvature in front of a convex lens, its image is formed at the centre of curvature on the other side of the lens. The image formed is real, inverted, and of the same size as the object.
Question 18: A spherical mirror and a thin spherical lens have each a focal length of
(a) both concave
(b) both convex
(c) the mirror is concave and the lens is convex
(d) the mirror is convex, but the lens is concave
Answer
(a) By convention, the focal length of a concave mirror and a concave lens are taken as negative. Hence, both the spherical mirror and the thin spherical lens are concave in nature.
Question 19: No matter how far you stand from a mirror, your image appears erect. The mirror is likely to be
(a) plane
(b) concave
(c) convex
(d) either plane or convex
Answer
(d) A convex mirror always gives a virtual and erect image of smaller size of the object placed in front of it. Similarly, a plane mirror will always give a virtual and erect image of same size as that of the object placed in front of it. Therefore, the given mirror could be either plane or convex. Answer
(d) A convex mirror always gives a virtual and erect image of smaller size of the object placed in front of it. Similarly, a plane mirror will always give a virtual and erect image of same size as that of the object placed in front of it. Therefore, the given mirror could be either plane or convex.
Question 20: Which of the following lenses would you prefer to use while reading small letters found in a dictionary?
(a) A convex lens of focal length
(b) A concave lens of focal length
(c) A convex lens of focal length
(d) A concave lens of focal length
Answer
(c) A convex lens gives a magnified image of an object when it is placed between the radius of curvature and focal length. Also, magnification is more for convex lenses having shorter focal length. Therefore, for reading small letters, a convex lens of focal length
Question 21: We wish to obtain an erect image of an object, using a concave mirror of focal length
Answer
Range of object distance
A concave mirror gives an erect image when an object is placed between its pole
Hence, to obtain an erect image of an object from a concave mirror of focal length
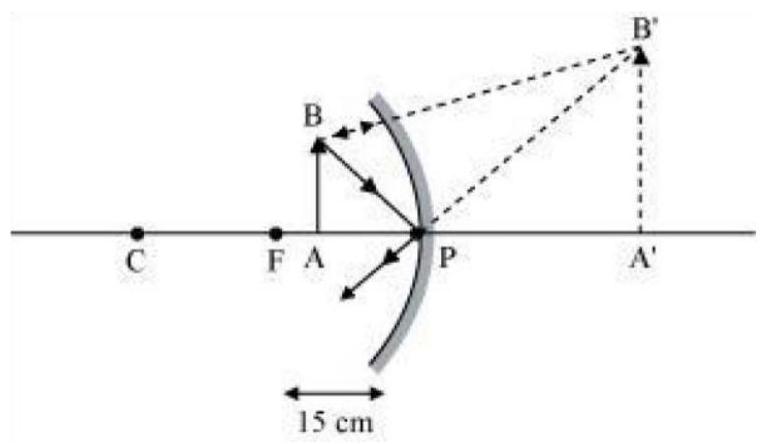
Question 19: Name the type of mirror used in the following situations.
(a) Headlights of a car
(b) Side/rear-view mirror of a vehicle
(c) Solar furnace
Support your Answer with reason.
Answer
(a) Concave (b) Convex (c) Concave
Explanation
(a) Concave mirror is used in the headlights of a car. This is because concave mirrors can produce powerful parallel beam of light when the light source is placed at their principal focus.
(b) Convex mirror is used in side/rear view mirror of a vehicle. Convex mirrors give a virtual, erect, and diminished image of the objects placed in front of it. Because of this, they have a wide field of view. It enables the driver to see most of the traffic behind him/her.
(c) Concave mirrors are convergent mirrors. That is why they are used to construct solar furnaces. Concave mirrors converge the light incident on them at a single point known as principal focus. Hence, they can be used to produce a large amount of heat at that point.
Question 20: One-half of a convex lens is covered with a black paper. Will this lens produce a complete image of the object? Verify your Answer experimentally. Explain your observations.
Answer
The convex lens will form complete image of an object, even if its one half is covered with black paper. It can be understood by the following two cases.
Case I
When the upper half of the lens is covered
In this case, a ray of light coming from the object will be refracted by the lower half of the lens. These rays meet at the other side of the lens to form the image of the given object, as shown in the following figure.

Case II
When the lower half of the lens is covered
In this case, a ray of light coming from the object is refracted by the upper half of the lens. These rays meet at the other side of the lens to form the image of the given object, as shown in the following figure.

Question 21: An object
Answer
Object distance,
Object height, ho
Focal length,
According to the lens formula,
The positive value of
Magnification,
The negative sign shows that the image is real and formed behind the lens.
Magnification,
The negative value of image height indicates that the image formed is inverted.
The position, size, and nature of image are shown in the following ray diagram.
Question 22: A concave lens of focal length
Answer
Focal length of concave lens (OF1),
Image distance,
According to the lens formula,
The negative value of
Question 23: An object is placed at a distance of
Answer
Focal length of convex mirror,
Object distance,
According to the mirror formula,
The positive value of
Magnification,
The positive value of magnification indicates that the image formed is virtual and erect.
13.
The magnification produced by a plane mirror is +1 . What does this mean?
Magnification produced by a mirror is given by the relation
Magnification,
The magnification produced by a plane mirror is +1 . It shows that the image formed by the plane mirror is of the same size as that of the object. The positive sign shows that the image formed is virtual and erect.
Question 24: An object
Answer
Object distance,
Object height,
Radius of curvature,
Radius of curvature
According to the mirror formula,
The positive value of
Magnification,
The positive value of magnification indicates that the image formed is virtual.
Magnification,
The positive value of image height indicates that the image formed is erect.
Therefore, the image formed is virtual, erect, and smaller in size.
Question 25: An object of size
Answer
Object distance,
Object height,
Focal length,
According to the mirror formula,
The screen should be placed at a distance of
Magnification,
The negative value of magnification indicates that the image formed is real.
Magnification,
The negative value of image height indicates that the image formed is inverted.
Question 26: Find the focal length of a lens of power -2.0 D. What type of lens is this?
Power of a lens,
Answer
A concave lens has a negative focal length. Hence, it is a concave lens.
Question 27: A doctor has prescribed a corrective lens of power +1.5 D. Find the focal length of the lens. Is the prescribed lens diverging or converging?
Power of a lens,
Power,
Answer
A convex lens has a positive focal length. Hence, it is a convex lens or a converging lens.
Human Eye and Colourful World
Question 1: What is meant by power of accommodation of the eye?
Answer
When the ciliary muscles are relaxed, the eye lens becomes thin, the focal length increases, and the distant objects are clearly visible to the eyes. To see the nearby objects clearly, the ciliary muscles contract making the eye lens thicker. Thus, the focal length of the eye lens decreases and the nearby objects become visible to the eyes. Hence, the human eye lens is able to adjust its focal length to view both distant and nearby objects on the retina. This ability is called the power of accommodation of the eyes.
A person with a myopic eye cannot see objects beyond
The person is able to see nearby objects clearly, but he is unable to see objects beyond

To correct this defect of vision, he must use a concave lens. The concave lens will bring the image back to the retina as shown in the given figure.

Question 2: What is the far point and near point of the human eye with normal vision?
Answer
The near point of the eye is the minimum distance of the object from the eye, which can be seen distinctly without strain. For a normal human eye, this distance is
The far point of the eye is the maximum distance to which the eye can see the objects clearly. The far point of the normal human eye is infinity.
Question 3: A student has difficulty reading the blackboard while sitting in the last row. What could be the defect the child is suffering from? How can it be corrected?
Answer
A student has difficulty in reading the blackboard while sitting in the last row. It shows that he is unable to see distant objects clearly. He is suffering from myopia. This defect can be corrected by using a concave lens.
Question 4: The human eye can focus objects at different distances by adjusting the focal length of the eye lens. This is due to
(a) presbyopia
(b) accommodation
(c) near-sightedness
(d) far-sightedness
Answer
(b) Human eye can change the focal length of the eye lens to see the objects situated at various distances from the eye. This is possible due to the power of accommodation of the eye lens.
Question 5: The human eye forms the image of an object at its
(a) cornea (b) iris (c) pupil (d) retina
Answer
(d) The human eye forms the image of an object at its retina.
Question 6: The least distance of distinct vision for a young adult with normal vision is about
(a)
(b)
(c)
(d)
Answer
(c) The least distance of distinct vision is the minimum distance of an object to see clear and distinct image. It is
Question 7: A person needs a lens of power -5.5 dioptres for correcting his distant vision. For correcting his near vision he needs a lens of power +1.5 dioptre. What is the focal length of the lens required for correcting (i) distant vision, and (ii) near vision?
Answer
For distant vision
The power
(i) Power of the lens used for correcting distant vision
Focal length of the required lens,
The focal length of the lens for correcting distant vision is
(ii) Power of the lens used for correcting near vision
Focal length of the required lens,
The focal length of the lens for correcting near vision is
Question 8: The far point of a myopic person is
Answer
The person is suffering from an eye defect called myopia. In this defect, the image is formed in front of the retina. Hence, a concave lens is used to correct this defect of vision.
Object distance,
Image distance,
Focal length
According to the lens formula,
We know,
Power,
A concave lens of power
Question 9: The far point of a myopic person is
Answer
The person is suffering from an eye defect called myopia. In this defect, the image is formed in front of the retina. Hence, a concave lens is used to correct this defect of vision.
Object distance,
Image distance,
Focal length
According to the lens formula,
We know,
Power,
A concave lens of power
Question 10: Make a diagram to show how hypermetropia is corrected. The near point of a hypermetropic eye is
Answer
A person suffering from hypermetropia can see distinct objects clearly but faces difficulty in seeing nearby objects clearly. It happens because the eye lens focuses the incoming divergent rays beyond the retina. This defect of vision is corrected by using a convex lens. A convex lens of suitable power converges the incoming light in such a way that the image is formed on the retina, as shown in the following figure.

Correction for hypermetropic eye
The convex lens actually creates a virtual image of a nearby object (
The given person will be able to clearly see the object kept at
Object distance,
Image distance,
Focal length,
Using the lens formula,
Power,
A convex lens of power
Question 11: Why is a normal eye not able to see clearly the objects placed closer than
Answer
A normal eye is unable to clearly see the objects placed closer than
If the object is placed at a distance less than
Question 12: What happens to the image distance in the eye when we increase the distance of an object from the eye?
Answer
Since the size of eyes cannot increase or decrease, the image distance remains constant. When we increase the distance of an object from the eye, the image distance in the eye does not change. The increase in the object distance is compensated by the change in the focal length of the eye lens. The focal length of the eyes changes in such a way that the image is always formed at the retina of the eye.
Question 13: Why do stars twinkle?
Answer
Stars emit their own light and they twinkle due to the atmospheric refraction of light. Stars are very far away from the earth. Hence, they are considered as point sources of light. When the light coming from stars enters the earth’s atmosphere, it gets refracted at different levels because of the variation in the air density at different levels of the atmosphere. When the star light refracted by the atmosphere comes more towards us, it appears brighter than when it comes less towards us. Therefore, it appears as if the stars are twinkling at night.
Question 14: Explain why the planets do not twinkle?
Answer
Planets do not twinkle because they appear larger in size than the stars as they are relatively closer to earth. Planets can be considered as a collection of a large number of point-size sources of light. The different parts of these planets produce either brighter or dimmer effect in such a way that the average of brighter and dimmer effect is zero. Hence, the twinkling effects of the planets are nullified and they do not twinkle.
Question 15: Why does the Sun appear reddish early in the morning?
Answer
During sunrise, the light rays coming from the Sun have to travel a greater distance in the earth’s atmosphere before reaching our eyes. In this journey, the shorter wavelengths of lights are scattered out and only longer wavelengths are able to reach our eyes. Since blue colour has a shorter wavelength and red colour has a longer wavelength, the red colour is able to reach our eyes after the atmospheric scattering of light. Therefore, the Sun appears reddish early in the morning.
Question 16: Why does the sky appear dark instead of blue to an astronaut?
Answer
The sky appears dark instead of blue to an astronaut because there is no atmosphere in the outer space that can scatter the sunlight. As the sunlight is not scattered, no scattered light reach the eyes of the astronauts and the sky appears black to them.
NCERT Solutions for Class 10th: Ch 12 Electricity Science
In Text Questions
Page No: 200
- What does an electric circuit mean?
Answer
A continuous and closed path of an electric current is called an electric circuit. An electric circuit consists of electric devices, source of electricity and wires that are connected with the help of a switch.
- Define the unit of current.
Answer
The unit of electric current is ampere (A).
- Calculate the number of electrons constituting one coulomb of charge.
Answer
One electron possesses a charge of
Therefore,
Page No: 202
- Name a device that helps to maintain a potential difference across a conductor.
Answer
Any source of electricity like battery, cell, power supply, etc. helps to maintain a potential difference across a conductor.
- What is meant by saying that the potential difference between two points is
Answer
If
- How much energy is given to each coulomb of charge passing through a
Answer
The energy given to each coulomb of charge is equal to the amount of work which is done in moving it.
Now we know that,
Potential difference
Where, Charge
Page No: 209
- On what factors does the resistance of a conductor depend?
Answer
The resistance of a conductor depends upon the following factors:
- Will current flow more easily through a thick wire or a thin wire of the same material, when connected to the same source? Why?
Answer
The current will flow more easily through thick wire. It is because the resistance of a conductor is inversely proportional to its area of cross - section. If thicker the wire, less is resistance and hence more easily the current flows.
- Let the resistance of an electrical component remains constant while the potential difference across the two ends of the component decreases to half of its former value. What change will occur in the current through it?
Answer
According to Ohm’s law
Now Potential difference is decreased to half
Resistance remains constant
So the new current
Therefore, the amount of current flowing through the electrical component is reduced by half.
- Why are coils of electric toasters and electric irons made of an alloy rather than a pure metal?
Answer
The resistivity of an alloy is higher than the pure metal. Moreover, at high temperatures, the alloys do not melt readily. Hence, the coils of heating appliances such as electric toasters and electric irons are made of an alloy rather than a pure metal.
- Use the data in Table 12.2 to Answer
the following -
Table 12.2 Electrical resistivity of some substances at
| - | Material | Resistivity |
|---|---|---|
| Conductors | Silver | |
| Copper | ||
| Aluminium | ||
| Tungsten | ||
| Nickel | ||
| Iron | ||
| Chromium | ||
| Mercury | ||
| Manganese | ||
| Constantan (alloy of Cu and Ni) |
||
| Alloys | Manganin (alloy of Cu, Mn and Ni) |
|
| :— | :— | :— |
| Nichrome (alloy of Ni, Cr, Mn and Fe) |
||
| Glass | ||
| Insulators | Hard rubber | |
| Ebonite | ||
| Diamond | ||
| Paper (dry) |
Answer
(a) Resistivity of iron
Resistivity of mercury
Resistivity of mercury is more than that of iron. This implies that iron is a better conductor than mercury.
(b) It can be observed from Table 12.2 that the resistivity of silver is the lowest among the listed materials. Hence, it is the best conductor.
Page No: 213
- Draw a schematic diagram of a circuit consisting of a battery of three cells of
Answer
Three cells of potential

- Redraw the circuit of question 1, putting in an ammeter to measure the current through the resistors and a voltmeter to measure potential difference across the
Answer
An ammeter should be connected in the circuit in series with the resistors. To measure the potential difference across the resistor it should be connected in parallel, as shown in the following figure.

The resistances are connected in series.
Ohm’s law can be used to obtain the readings of ammeter and voltmeter. According to Ohm’s law,
Where,
Potential difference,
Current flowing through the circuit/resistors
Resistance of the circuit,
Potential difference across
Current flowing through the
Therefore, using Ohm’s law, we obtain
Therefore, the reading of the ammeter will be
The reading of the voltmeter will be
- Judge the equivalent resistance when the following are connected in parallel - (a)
Answer
(a) When
Let
Therefore, equivalent resistance
(b) When
Let
Therefore, equivalent resistance
- An electric lamp of
www.ncrtsolutions.in through it?
Answer
Resistance of electric lamp,
Resistance of toaster,
Resistance of water filter,
Potential difference of the source,
These are connected in parallel, as shown in the following figure.
Let
According to Ohm’s law,
Where,
Current flowing through the circuit
7.04 A of current is drawn by all the three given appliances.
Therefore, current drawn by an electric iron connected to the same source of potential
Let
Therefore, the resistance of the electric iron is
- What are the advantages of connecting electrical devices in parallel with the battery instead of connecting them in series?
Answer
There is no division of voltage among the appliances when connected in parallel. The potential difference across each appliance is equal to the supplied voltage.
The total effective resistance of the circuit can be reduced by connecting electrical appliances in parallel.
- How can three resistors of resistances
Answer
There are three resistors of resistances
(a) The following circuit diagram shows the connection of the three resistors.
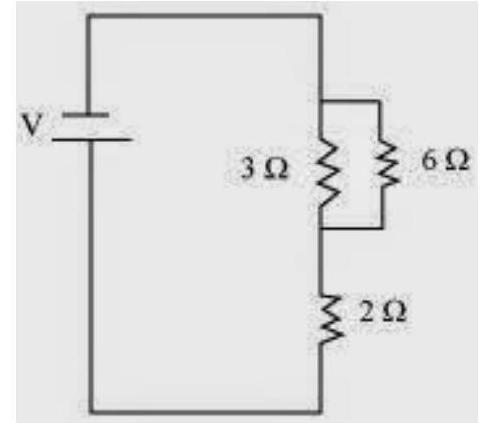
Here,
Therefore, their equivalent resistance will be given by
This equivalent resistor of resistance
Therefore, the equivalent resistance of the circuit
Hence the total resistance of the circuit is
(b) The following circuit diagram shows the connection of the three resistors.
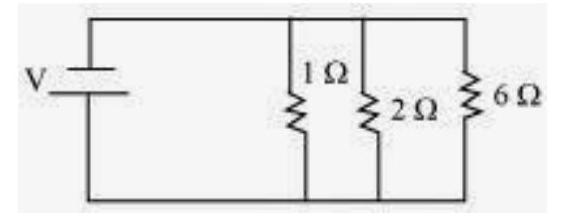
All the resistors are connected in series. Therefore, their equivalent resistance will be given as
Therefore, the total resistance of the circuit is
- What is (a) the highest, (b) the lowest total resistance that can be secured by combinations of four coils of resistance
Answer
There are four coils of resistances
(a) If these coils are connected in series, then the equivalent resistance will be the highest, given by the sum
(b) If these coils are connected in parallel, then the equivalent resistance will be the lowest, given by
Therefore,
Page No: 218
- Why does the cord of an electric heater not glow while the heating element does?
Answer
The heating element of the heater is made up of alloy which has very high resistance so when current flows through the heating element, it becomes too hot and glows red. But the resistance of cord which is usually of copper or aluminium is very law so it does not glow.
- Compute the heat generated while transferring 96000 coulomb of charge in one hour through a potential difference of
Answer
Given Charge,
Time,
Potential difference,
Now we know that
So we have to calculate / first
As
Therefore, the heat generated is
- An electric iron of resistance
Answer
The amount of heat
Time,
Voltage,
Therefore, the amount of heat developed in the electric iron is
Page No: 220
- What determines the rate at which energy is delivered by a current?
Answer
The rate of consumption of electric energy in an electric appliance is called electric power. Hence, the rate at which energy is delivered by a current is the power of the appliance.
- An electric motor takes
Answer
Power
Where,
Voltage,
Current,
Energy consumed by the motor
Where,
Time,
Therefore, power of the motor
Energy consumed by the motor
Page No: 221
Excercise
- A piece of wire of resistance
(b)
(c) 5
(d) 25
(d) 25
- Which of the following terms does not represent electrical power in a circuit?
(a)
(b)
(c)
(d)
(b)
- An electric bulb is rated
(a)
(b)
(c)
(d)
(d)
- Two conducting wires of the same material and of equal lengths and equal diameters are first connected in series and then parallel in a circuit across the same potential difference. The ratio of heat produced in series and parallel combinations would be -
(a)
(b)
(c)
(d)
(c)
- How is a voltmeter connected in the circuit to measure the potential difference between two points?
Answer
To measure the potential difference between two points, a voltmeter should be connected in parallel to the points.
- A copper wire has diameter
Answer
Area of cross-section of the wire,
Diameter
Resistance,
We know that
If the diameter of the wire is doubled, new diameter
Therefore, the length of the wire is
- The values of current / flowing in a given resistor for the corresponding values of potential difference
Plot a graph between
Answer
The plot between voltage and current is called
| 1.6 | 3.4 | 6.7 | 10.2 | 13.2 | |
|---|---|---|---|---|---|
| 0.5 | 1.0 | 2.0 | 3.0 | 4.0 |
The IV characteristic of the given resistor is plotted in the following figure.

The slope of the line gives the value of resistance
Slope
Therefore, the resistance of the resistor is
- When a
Answer
Resistance
Where,
Potential difference,
Current in the circuit,
Therefore, the resistance of the resistor is
- A battery of
Answer
There is no current division occurring in a series circuit. Current flow through the component is the same, given by Ohm’s law as
Where,
Potential difference,
Therefore, the current that would flow through the
- How many
Answer
For
Where,
Supply voltage,
Current,
Equivalent resistance of the combination
From Ohm’s law,
Therefore, four resistors of
- Show how you would connect three resistors, each of resistance
Answer
If we connect the resistors in series, then the equivalent resistance will be the sum of the resistors, i.e.,
(a) Two resistor in parallel

Two
The third
(b) Two resistor in series

Two
Therefore, the total resistance is
- Several electric bulbs designed to be used on a
Answer
Resistance
Supply voltage,
Maximum allowable current,
Rating of an electric bulb
Because
According to Ohm’s law,
Let
Resistance of each electric bulb,
- A hot plate of an electric oven connected to a
Answer
Supply voltage,
Resistance of one coil,
(i) Coils are used separately
According to Ohm’s law,
Where,
Therefore, 9.16 A current will flow through the coil when used separately.
(ii) Coils are connected in series
Total resistance,
According to Ohm’s law,
Where,
Therefore,
(iii) Coils are connected in parallel
Total resistance,
According to Ohm’s law,
Where,
Therefore, 18.33 A current will flow through the circuit when coils are connected in parallel.
- Compare the power used in the
Answer
(i) Potential difference,
According to Ohm’s law,
Where,
This current will flow through each component of the circuit because there is no division of current in series circuits. Hence, current flowing through the
(ii) Potential difference,
Power consumed by
Therefore, the power used by
- Two lamps, one rated
Answer
Both the bulbs are connected in parallel. Therefore, potential difference across each of them will be
Current drawn by the bulb of rating
Current
Hence, current drawn from the line
- Which uses more energy, a
Answer
Energy consumed by an electrical appliance is given by the expression,
Where,
Power of the appliance
Time
Energy consumed by a TV set of power
Energy consumed by a toaster of power
Energy consumed by a toaster of power
Therefore, the energy consumed by a
- An electric heater of resistance
Answer
Rate of heat produced by a device is given by the expression for power as,
Current drawn,
Therefore, heat is produced by the heater at the rate of
- Explain the following.
(a) Why is the tungsten used almost exclusively for filament of electric lamps?
(b) Why are the conductors of electric heating devices, such as bread-toasters and electric irons, made of an alloy rather than a pure metal?
(c) Why is the series arrangement not used for domestic circuits?
(d) How does the resistance of a wire vary with its area of cross-section?
(e) Why are copper and aluminium wires usually employed for electricity transmission?
Answer
(a) The melting point and of Tungsten is an alloy which has very high melting point and very high resistivity so does not burn easily at a high temperature.
(b) The conductors of electric heating devices such as bread toasters and electric irons are made of alloy because resistivity of an alloy is more than that of metals which produces large amount of heat.
(c) In series circuits voltage is divided. Each component of a series circuit receives a small voltage so the amount of current decreases and the device becomes hot and does not work properly. Hence, series arrangement is not used in domestic circuits.
(d) Resistance (R) of a wire is inversely proportional to its area of cross-section (A), i.e. when area of cross section increases the resistance decreases or vice versa.
(e) Copper and aluminium are good conductors of electricity also they have low resistivity. So they are usually used for electricity transmission.
Magnetic Effects of Electric Current
Question 1: Why does a compass needle get deflected when brought near a bar magnet?
Answer
A compass needle is a small bar magnet. When it is brought near a bar magnet, its magnetic field lines interact with that of the bar magnet. Hence, a compass needle shows a deflection when brought near the bar magnet.
Question 2: Draw magnetic field lines around a bar magnet.
Answer
Magnetic field lines of a bar magnet emerge from the north pole and terminate at the south pole. Inside the magnet, the field lines emerge from the south pole and terminate at the north pole, as shown in the given figure.

Question 3: List the properties of magnetic lines of force.
Answer
The properties of magnetic lines of force are as follows.
(a) Magnetic field lines emerge from the north pole.
(b) They merge at the south pole.
(c) The direction of field lines inside the magnet is from the south pole to the north pole.
(d) Magnetic lines do not intersect with each other.
Question 4: Why don’t two magnetic lines of force intersect each other?
Answer
If two field lines of a magnet intersect, then at the point of intersection, the compass needle points in two different directions. This is not possible. Hence, two field lines do not intersect each other.
Question 5: Consider a circular loop of wire lying in the plane of the table. Let the current pass through the loop clockwise. Apply the right-hand rule to find out the direction of the magnetic field inside and outside the loop.
Answer
Inside the loop = Pierce inside the table
Outside the loop = Appear to emerge out from the table
For downward direction of current flowing in the circular loop, the direction of magnetic field lines will be as if they are emerging from the table outside the loop and merging in the table inside the loop. Similarly, for upward direction of current flowing in the circular loop, the direction of magnetic field lines will be as if they are emerging from the table outside the loop and merging in the table inside the loop, as shown in the given figure.

Question 6: The magnetic field in a given region is uniform. Draw a diagram to represent it.

The magnetic field lines inside a current-carrying long straight solenoid are uniform.
Question 7: Consider a circular loop of wire lying in the plane of the table. Let the current pass through the loop clockwise. Apply the right-hand rule to find out the direction of the magnetic field inside and outside the loop.
Answer
Inside the loop = Pierce inside the table
Outside the loop = Appear to emerge out from the table
For downward direction of current flowing in the circular loop, the direction of magnetic field lines will be as if they are emerging from the table outside the loop and merging in the table inside the loop. Similarly, for upward direction of current flowing in the circular loop, the direction of magnetic field lines will be as if they are emerging from the table outside the loop and merging in the table inside the loop, as shown in the given figure.

Question 8:
The magnetic field in a given region is uniform. Draw a diagram to represent it.
Answer

The magnetic field lines inside a current-carrying long straight solenoid are uniform.
Question 9: Choose the correct option.
The magnetic field inside a long straight solenoid-carrying current
(a) is zero
(b) decreases as we move towards its end
(c) increases as we move towards its end
(d) is the same at all points
Answer
(d) The magnetic field inside a long, straight, current-carrying solenoid is uniform. It is the same at all points inside the solenoid.
Question 10: C while it moves freely in a magnetic field? (There may be more than one correct answer.)
(a) mass
(b) speed
(c) velocity
(d) momentum
Answer
(c) and (d)
When a proton enters in a region of magnetic field, it experiences a magnetic force. As a result of the force, the path of the proton becomes circular. Hence, its velocity and momentum change.
Question 11: State Fleming’s left-hand rule.
Answer
Fleming’s left hand rule states that if we arrange the thumb, the centre finger, and the forefinger of the left hand at right angles to each other, then the thumb points towards the direction of the magnetic force, the centre finger gives the direction of current, and the forefinger points in the direction of magnetic field.
Question 12: What is the principle of an electric motor?
Answer
The working principle of an electric motor is based on the magnetic effect of current. A current-carrying loop experiences a force and rotates when placed in a magnetic field. The direction of rotation of the loop is given by the Fleming’s left-hand rule.
Question 13: What is the role of the split ring in an electric motor?
Answer
The split ring in the electric motor acts as a commutator. The commutator reverses the direction of current flowing through the coil after each half rotation of the coil. Due to this reversal of the current, the coil continues to rotate in the same direction.
Question 14: Explain different ways to induce current in a coil.
Answer
The different ways to induce current in a coil are as follows:
(a) If a coil is moved rapidly between the two poles of a horse-shoe magnet, then an electric current is induced in the coil.
(b) If a magnet is moved relative to a coil, then an electric current is induced in the coil.
Question 15: State the principle of an electric generator.
Answer
An electric generator works on the principle of electromagnetic induction. It generates electricity by rotating a coil in a magnetic field.
Question 16: Name some sources of direct current.
Answer
Some sources of direct current are cell, DC generator, etc.
Question 17: Which sources produce alternating current?
Answer
AC generators, power plants, etc., produce alternating current.
Question 18: Choose the correct option.
Answer
A rectangular coil of copper wires is rotated in a magnetic field. The direction of the induced current changes once in each
(a) two revolutions(b) one revolution
(c) half revolution (d) one-fourth revolution
(c) When a rectangular coil of copper is rotated in a magnetic field, the direction of the induced current in the coil changes once in each half revolution. As a result, the direction of current in the coil remains the same.
Question 19: Name two safety measures commonly used in electric circuits and appliances.
Answer
Two safety measures commonly used in electric circuits and appliances are as follows:
(i) Each circuit must be connected with an electric fuse. This prevents the flow of excessive current through the circuit. When the current passing through the wire exceeds the maximum limit of the fuse element, the fuse melts to stop the flow of current through that circuit, hence protecting the appliances connected to the circuit.
(ii) Earthing is a must to prevent electric shocks. Any leakage of current in an electric appliance is transferred to the ground and people using the appliance do not get the shock.
Question 20: An electric oven of
Answer
Current drawn by the electric oven can be obtained by the expression,
Where,
Current
Power of the oven,
Voltage supplied,
Hence, the current drawn by the electric oven is
Question 21: What precaution should be taken to avoid the overloading of domestic electric circuits?
Answer
The precautions that should be taken to avoid the overloading of domestic circuits are as follows:
(a) Too many appliances should not be connected to a single socket.
(b) Too many appliances should not be used at the same time.
(c) Faulty appliances should not be connected in the circuit.
(d) Fuse should be connected in the circuit.
Question 22: Which of the following correctly describes the magnetic field near a long straight wire?
(a) The field consists of straight lines perpendicular to the wire
(b) The field consists of straight lines parallel to the wire
(c) The field consists of radial lines originating from the wire
(d) The field consists of concentric circles centred on the wire
Answer
(d) The magnetic field lines, produced around a straight current-carrying conductor, are concentric circles. Their centres lie on the wire.
Question 23: The phenomenon of electromagnetic induction is
(a) the process of charging a body
(b) the process of generating magnetic field due to a current passing through a coil
(c) producing induced current in a coil due to relative motion between a magnet and the coil
(d) the process of rotating a coil of an electric motor
Answer
(c) When a straight coil and a magnet are moved relative to each other, a current is induced in the coil. This phenomenon is known as electromagnetic induction.
Question 24: The device used for producing electric current is called a
(a) generator
(b) galvanometer
(c) ammeter
(d) motor
Answer
(a) An electric generator produces electric current. It converts mechanical energy into electricity.
Question 25: The essential difference between an AC generator and a DC generator is that
(a)
(b) DC generator will generate a higher voltage.
(c) AC generator will generate a higher voltage.
(d) AC generator has slip rings while the DC generator has a commutator.
Answer
(d) An AC generator has two rings called slip rings. A DC generator has two half rings called commutator. This is the main difference between both the types of generators.
Question 26: At the time of short circuit, the current in the circuit
(a) reduces substantially
(b) does not change
(c) increases heavily
(d) vary continuously
Answer
(c) When two naked wires of an electric circuit touch each other, the amount of current that is flowing in the circuit increases abruptly. This causes short-circuit.
Question 27: State whether the following statements are true or false.
(a) An electric motor converts mechanical energy into electrical energy.
(b) An electric generator works on the principle of electromagnetic induction.
(c) The field at the centre of a long circular coil carrying current will be parallel straight lines.
(d) A wire with a green insulation is usually the live wire of an electric supply.
Answer
(a) False
An electric motor converts electrical energy into mechanical energy.
(b) True
A generator is an electric device that generates electricity by rotating a coil in a magnetic field. It works on the principle of electromagnetic induction.
(c) True
A long circular coil is a long solenoid. The magnetic field lines inside the solenoid are parallel lines.
(d) False
Live wire has red insulation cover, whereas earth wire has green insulation colour in the domestic circuits.
Question 28: List three sources of magnetic fields.
Answer
Three sources of magnetic fields are as follows:
(a) Current-carrying conductors
(b) Permanent magnets
(c) Electromagnets
Question 29: How does a solenoid behave like a magnet? Can you determine the north and south poles of a current-carrying solenoid with the help of a bar magnet? Explain.
Answer
A solenoid is a long coil of circular loops of insulated copper wire. Magnetic field lines are produced around the solenoid when a current is allowed to flow through it. The magnetic field produced by it is similar to the magnetic field of a bar magnet. The field lines produced in a currentcarrying solenoid is shown in the following figure.

In the above figure, when the north pole of a bar magnet is brought near the end connected to the negative terminal of the battery, the solenoid repels the bar magnet. Since like poles repel each other, the end connected to the negative terminal of the battery behaves as the north pole of the solenoid and the other end behaves as a south pole. Hence, one end of the solenoid behaves as a north pole and the other end behaves as a south pole.
Question 30: When is the force experienced by a current-carrying conductor placed in a magnetic field largest?
Answer
The force experienced by a current-currying conductor is the maximum when the direction of current is perpendicular to the direction of the magnetic field.
Question 31: Imagine that you are sitting in a chamber with your back to one wall. An electron beam, moving horizontally from back wall towards the front wall, is deflected by a strong magnetic field to your right side. What is the direction of magnetic field?
Answer
The direction of magnetic field is given by Fleming’s left hand rule. Magnetic field inside the chamber will be perpendicular to the direction of current (opposite to the direction of electron) and direction of deflection/force i.e., either upward or downward. The direction of current is from the front wall to the back wall because negatively charged electrons are moving
from back wall to the front wall. The direction of magnetic force is rightward. Hence, using Fleming’s left hand rule, it can be concluded that the direction of magnetic field inside the chamber is downward.
Question 32: Imagine that you are sitting in a chamber with your back to one wall. An electron beam, moving horizontally from back wall towards the front wall, is deflected by a strong magnetic field to your right side. What is the direction of magnetic field?
Answer
The direction of magnetic field is given by Fleming’s left hand rule. Magnetic field inside the chamber will be perpendicular to the direction of current (opposite to the direction of electron) and direction of deflection/force i.e., either upward or downward. The direction of current is from the front wall to the back wall because negatively charged electrons are moving from back wall to the front wall. The direction of magnetic force is rightward. Hence, using Fleming’s left hand rule, it can be concluded that the direction of magnetic field inside the chamber is downward.
Question 33: Draw a labelled diagram of an electric motor. Explain its principle and working. What is the function of a split ring in an electric motor?
Answer
An electric motor converts electrical energy into mechanical energy.
It works on the principle of the magnetic effect of current. A current-carrying coil rotates in a magnetic field. The following figure shows a simple electric motor.

When a current is allowed to flow through the coil MNST by closing the switch, the coil starts rotating anti-clockwise. This happens because a downward force acts on length
Current in the length
After half a rotation, the position of MN and ST interchange. The half-ring D comes in contact with brush

The current flows through the coil in the direction TSNM. The reversal of current through the coil MNST repeats after each half rotation. As a result, the coil rotates unidirectional. The split rings help to reverse the direction of current in the circuit. These are called the commutator.
Question 34: Name some devices in which electric motors are used?
Answer
Some devices in which electric motors are used are as follows:
(a) Water pumps
(b) Electric fans
(c) Electric mixers
(d) Washing machines
Question 35: A coil of insulated copper wire is connected to a galvanometer. What will happen if a bar magnet is (i) pushed into the coil, (ii) withdrawn from inside the coil, (iii) held stationary inside the coil?
Answer
A current induces in a solenoid if a bar magnet is moved relative to it. This is the principle of electromagnetic induction.
(i) When a bar magnet is pushed into a coil of insulated copper wire, a current is induced momentarily in the coil. As a result, the needle of the galvanometer deflects momentarily in a particular direction.
(ii) When the bar magnet is withdrawn from inside the coil of the insulated copper wire, a current is again induced momentarily in the coil in the opposite direction. As a result, the needle of the galvanometer deflects momentarily in the opposite direction.
(iii) When a bar magnet is held stationary inside the coil, no current will be induced in the coil. Hence, galvanometer will show no deflection.
Question 36: Two circular coils A and B are placed closed to each other. If the current in the coil
Answer
Two circular coils A and B are placed close to each other. When the current in coil A is changed, the magnetic field associated with it also changes. As a result, the magnetic field around
coil B also changes. This change in magnetic field lines around coil B induces an electric current in it. This is called electromagnetic induction.
Question 37: State the rule to determine the direction of a (i) magnetic field produced around a straight conductor-carrying current, (ii) force experienced by a current-carrying straight conductor placed in a magnetic field which is perpendicular to it, and (iii) current induced in a coil due to its rotation in a magnetic field.
Answer
(i) Maxwell’s right hand thumb rule
(ii) Fleming’s left hand rule
(iii) Fleming’s right hand rule
Question 38: Explain the underlying principle and working of an electric generator by drawing a labelled diagram. What is the function of brushes?
Answer
An electric generator converts mechanical energy into electrical energy.
The principle of working of an electric generator is that when a loop is moved in a magnetic field, an electric current is induced in the coil. It generates electricity by rotating a coil in a magnetic field. The following figure shows a simple AC generator.

MNST
If axle Xis rotated clockwise, then the length MN moves upwards while length ST moves downwards. Since the lengths MN and ST are moving in a magnetic field, a current will be
induced in both of them due to electromagnetic induction. Length
The direction of current in the coil is MNST. Hence, the galvanometer shows a deflection in a particular direction. After half a rotation, length MN starts moving down whereas length ST starts moving upward. The direction of the induced current in the coil gets reversed as TSNM. As the direction of current gets reversed after each half rotation, the produced current is called an alternating current (AC).
To get a unidirectional current, instead of two slip rings, two split rings are used, as shown in the following figure.

In this arrangement, brush A always remains in contact with the length of the coil that is moving up whereas brush B always remains in contact with the length that is moving down. The split rings
The direction of current induced in the coil will be MNST for the first rotation and TSNM in the second half of the rotation. Hence, a unidirectional current is produced from the generator called DC generator. The current is called AC current.
Question 39: When does an electric short circuit occur?
Answer
If the resistance of an electric circuit becomes very low, then the current flowing through the circuit becomes very high. This is caused by connecting too many appliances to a single socket or connecting high power rating appliances to the light circuits. This results in a short circuit.
When the insulation of live and neutral wires undergoes wear and tear and then touches each other, the current flowing in the circuit increases abruptly. Hence, a short circuit occurs.
Question 40: What is the function of an earth wire? Why is it necessary to earth metallic appliances?
Answer
The metallic body of electric appliances is connected to the earth by means of earth wire so that any leakage of electric current is transferred to the ground. This prevents any electric shock to the user. That is why earthing of the electrical appliances is necessary.
Sources of Energy
Question 1: What is a good source of energy?
Answer
A good source of energy fulfils the following criteria:
(I) It produces a lot of heat per unit mass.
(II) It does a huge amount of work per unit mass.
(III) It is easily accessible.
(IV) It is easy to store and transport.
(V) It is economical.
(VI) It produces less amount of smoke.
Question 2: What is a good fuel?
Answer
A good fuel produces a huge amount of heat on burning, does not produce a lot of smoke, and is easily available.
Question 3: If you could use any source of energy for heating your food, which one would you use and why?
Answer
Natural gas can be used for heating and cooking food because it is a clean source of energy. It does not produce huge amount of smoke on burning. Although it is highly inflammable, it is easy to use, transport, and it produces a huge amount of heat on burning.
Question 4: What are the disadvantages of fossil fuels?
Answer
The disadvantages of fossil fuels are as follows:
(a) Burning of coal and petroleum produces a lot of pollutants causing air pollution.
(b) Fossil fuels release oxides of carbon, nitrogen, sulphur, etc. that cause acid rain, which affects the soil fertility and potable water.
(c) Burning of fossil fuels produce gases such as carbon dioxide that causes global warming.
Question 5: Why are we looking at alternate sources of energy?
Answer
Fossil fuels, which have been traditionally used by human beings as an energy sources, are non-renewable sources of energy. These sources of energy are limited and cannot replenish on their own. They are being consumed at a large rate. If this rate of consumption continues, then the fossil fuels would be exhausted from the Earth. Therefore, we have to conserve the energy sources. Hence, we should look for alternate sources of energy.
Question 3: How has the traditional use of wind and water energy been modified for our convenience?
Answer
Traditionally, waterfalls were used as a source of potential energy which was converted to electricity with the help of turbines. Since waterfalls are few in number, water dams have been constructed in large numbers. Nowadays, hydro-dams are used in order to harness potential energy of stored water. In water dams, water falls from a height on the turbine, which produces electricity.
Earlier, the windmills were used to harness wind energy to do mechanical work such as lifting/drawing water from a well. Today, windmills are used to generate electricity. In windmills, the kinetic energy of wind is harnessed and converted into electricity. The rotatory motion of the blades turns the turbine of the electric generator to generate electricity.
Question 4: What kind of mirror - concave, convex or plain - would be best suited for use in a solar cooker? Why?
Answer
A solar cooker uses heat of the sunlight to cook and heat food. A mirror is used in order to reflect and focus sunlight at a point. A concave mirror is used in a solar cooker for this purpose. The mirror focuses all the incident sunlight at a point. The temperature at that point increases, thereby cooking and heating the food placed at that point.
Question 5: What are the limitations of the energy that can be obtained from the oceans?
Answer
The forms of energy that can be obtained from the ocean are tidal energy, wave energy, and ocean thermal energy. There are several limitations in order to harness these energies.
(i) Tidal energy depends on the relative positioning of the Earth, moon, and the Sun.
(ii) High dams are required to be built to convert tidal energy into electricity.
(iii) Very strong waves are required to obtain electricity from wave energy.
(iv) To harness ocean thermal energy efficiently, the difference in the temperature of surface water (hot) and the water at depth (cold) must be
Question 6: What is geothermal energy?
Answer
Geothermal power plants use heat of the Earth to generate electricity. This heat energy of the Earth is known as geothermal energy.
When there are geological changes, the molten rocks present in the core of the earth are pushed to the earth’s crust. This forms regions of hot spot. Steam is generated when the underground water comes in contact with these hot spots forming hot springs. This trapped steam is used to generate electricity in the geothermal power plants.
Question 7: What are the advantages of nuclear energy?
Answer
The advantages of nuclear energy are as follows:
(a) Large amount of energy is produced per unit mass.
(b) It does not produce smoke. It is a clean energy.
(c) Fission of one atom of uranium produces 10 million times the energy released by burning of one atom of carbon.
(d) Fusion of four hydrogen atoms produces huge amount of energy approximately equal to 27
Question 8: Can any source of energy be pollution-free? Why or why not?
Answer
No source of energy can be pollution-free. It is considered that solar cells are pollutionfree. However, even their making causes environmental damage indirectly.
Also, in the case of nuclear energy, there is no waste produced after the fusion reactions. However, it is not totally pollution-free. To start the fusion reactions, approximately
Question 9: Hydrogen has been used as a rocket fuel. Would you consider it a cleaner fuel than CNG? Why or why not?
Answer
Hydrogengas is cleaner than CNG. CNG contains hydrocarbons. Therefore, it has carbon contents. Carbon is a form of pollutant present in CNG. On the other hand, hydrogen is waste-free. The fusion of hydrogen does not produce any waste. Hence, hydrogen is cleaner than CNG.
Question 10: Name two energy sources that you would consider to be renewable. Give reasons for your choices.
Answer
Two renewable sources of energy are as follows:
(a) Sun: The energy derived from the Sun is known as solar energy. Solar energy is produced by the fusion of hydrogen into helium, fusion of helium into other heavy elements, and so on. A large amount of hydrogen and helium is present in the Sun. Therefore, solar energy can replenish on its own. The Sun has 5 billion years more to burn. Hence, solar energy is a renewable source of energy.
(b) Wind: Wind energy is derived from air blowing with high speed. Wind energy is harnessed by windmills in order to generate electricity. Air blows because of uneven heating of the Earth. Since the heating of the Earth will continue forever, wind energy will also be available forever.
Question 11: Give the names of two energy sources that you would consider to be exhaustible. Give reasons for your choices.
Answer
Two exhaustible energy sources are as follows:
(a) Coal: It is produced from dead remains of plants and animals that remain buried under the earth’s crust for millions of years. It takes millions of years to produce coal. Industrialization has increased the demand of coal. However, coal cannot replenish within a short period of time. Hence, it is a non-renewable or exhaustible source of energy.
(b) Wood: It is obtained from forests. Deforestation at a faster rate has caused a reduction in the number of forests on the Earth. It takes hundreds of years to grow a forest. If deforestation is continued at this rate, then there would be no wood left on the Earth. Hence, wood is an exhaustible source of energy.
Question 12:
(a) a sunny day
(b) a cloudy day
(c) a hot day
(d) a windy day
Answer
(b)
Abstract
A solar water heater uses solar energy to heat water. It requires bright and intense sunlight to function properly. On a cloudy day, the sunlight reflects back in the sky from the clouds and is unable to reach the ground. Therefore, solar energy is not available for the solar heater to work properly. Hence, solar water heater does not function on a cloudy day.
Question 13: A solar water heater cannot be used to get hot water on
(a) a sunny day (b) a cloudy day
(c) a hot day (d) a windy day
Answer
(b) A solar water heater uses solar energy to heat water. It requires bright and intense sunlight to function properly. On a cloudy day, the sunlight reflects back in the sky from the clouds and is unable to reach the ground. Therefore, solar energy is not available for the solar heater to work properly. Hence, solar water heater does not function on a cloudy day.
Question 14: Which of the following is not an example of a bio-mass energy source?
(a) wood (b) gobar gas
(c) nuclear energy(d) coal
Answer
(c) Bio-mass is a source of energy that is obtained from plant materials and animal wastes. Nuclear energy is released during nuclear fission and fusion. In nuclear fission, uranium atom is bombarded with low-energy neutrons. Hence, uranium atom splits into two relatively lighter nuclei. This reaction produces huge amount of energy. In nuclear fusion reaction, lighter nuclei are fused together to form a relatively heavier nuclei. This reaction produces tremendous amount of energy. Hence, nuclear energy is not an example of bio-mass energy source.
Wood is a plant material, gobar gas is formed from animal dung, and coal is a fossil fuel obtained from the buried remains of plants and animals. Hence, these are bio-mass products.
Question 15: Most of the sources of energy we use represent stored solar energy. Which of the following is not ultimately derived from the Sun’s energy?
(a) Geothermal energy
(b) Wind energy
(c) Nuclear energy
(d) Bio-mass
Answer
(c) Nuclear energy is released during nuclear fission and fusion. In nuclear fission, uranium atom is bombarded with low-energy neutrons. Hence, uranium atom splits into two relatively lighter nuclei. This reaction produces huge amount of energy. In nuclear fusion reaction, lighter nuclei are fused together to form a relatively heavier nuclei. The energy required to fuse the lighter nuclei is provided by fission reactions. This reaction produces tremendous amount of energy. These reactions can be carried out in the absence or presence of sunlight. There is no effect of sunlight on these reactions. Hence, nuclear energy is not ultimately derived from Sun’s energy.
Geothermal energy, wind energy, and bio-mass are all ultimately derived from solar energy.
Geothermal energy is stored deep inside the earth’s crust in the form of heat energy. The heating is caused by the absorption of atmospheric and oceanic heat. It is the sunlight that heats the atmosphere and oceans.
Wind energy is harnessed from the blowing of winds. The uneven heating of the earth’s surface by the Sun causes wind.
Bio-mass is derived from dead plants and animal wastes. Chemical changes occur in these dead plants and animal wastes in the presence of water and sunlight. Hence, bio-mass is indirectly related to sunlight.
Question 16: Compare and contrast fossil fuels and the Sun as direct sources of energy.
Answer
Fossil fuels are energy sources, such as coal and petroleum, obtained from underneath the Earth’s crust. They are directly available to human beings for use. Hence, fossil fuels are the direct source of energy. These are limited in amount. These are non-renewable sources of energy because these cannot be replenished in nature. Fossil fuels take millions of years for their formation. If the present fossil fuel of the Earth gets exhausted, its formation will take several years. Fossil fuels are also very costly.
On the other hand, solar energy is a renewable and direct source of energy. The Sun has been shining for several years and will do so for the next five billion years. Solar energy is available free of cost to all in unlimited amount. It replenishes in the Sun itself.
Question 17: Compare and contrast bio-mass and hydro electricity as sources of energy.
Answer
Bio-mass and hydro-electricity both are renewable sources of energy. Bio-mass is derived from dead plants and animal wastes. Hence, it is naturally replenished. It is the result of natural processes. Wood, gobar gas, etc. are some of the examples of bio-mass.
Hydro-electricity, on the other hand, is obtained from the potential energy stored in water at a height. Energy from it can be produced again and again. It is harnessed from water and obtained from mechanical processes.
Question 18: What are the limitations of extracting energy from -
WwW.ncrtsolutions.in
(a) the wind? (b) waves? (c) tides?
Answer
(a) Wind energy is harnessed by windmills. One of the limitations of extracting energy from wind is that a windmill requires wind of speed more than
(b) Very strong ocean waves are required in order to extract energy from waves.
(c) Very high tides are required in order to extract energy from tides. Also, occurrence of tides depends on the relative positions of the Sun, moon, and the Earth.
Question 19: On what basis would you classify energy sources as
(a) renewable and non-renewable?
(b) exhaustible and inexhaustible?
Are the options given in (a) and (b) the same?
Answer
(a) The source of energy that replenishes in nature is known as renewable source of energy. Sun, wind, moving water, bio-mass, etc. are some of the examples of renewable sources of energy.
The source of energy that does not replenish in nature is known as non-renewable source of energy. Coal, petroleum, natural gas, etc. are some of the examples of non-renewable sources of energy.
(b) Exhaustible sources are those sources of energy, which will deplete and exhaust after a few hundred years. Coal, petroleum, etc. are the exhaustible sources of energy.
Inexhaustible resources of energy are those sources, which will not exhaust in future. These are unlimited. Bio-mass is one of the inexhaustible sources of energy.
Yes. The options given in (a) and (b) are the same.
Question 20: What are the qualities of an ideal source of energy?
An ideal source of energy must be:
I. Economical
II. Easily accessible
III. Smoke/pollution free
IV. Easy to store and transport
V. Able to produce huge amount of heat and energy on burning
Question 21: What are the advantages and disadvantages of using a solar cooker? Are there places where solar cookers would have limited utility?
Answer
Solar cooker uses Sun’s energy to heat and cook food. It is inexhaustible and clean renewable source of energy. It is free for all and available in unlimited amount. Hence, operating a solar cooker is not expensive.
Disadvantage of a solar cooker is that it is very expensive. It does not work without sunlight. Hence, on cloudy day, it becomes useless.
The places where the days are too short or places with cloud covers round the year, have limited utility for solar cooker.
Question 22: What are the environmental consequences of the increasing demand for energy? What steps would you suggest to reduce energy consumption?
Answer
Industrialization increases the demand for energy. Fossil fuels are easily accessible sources of energy that fulfil this demand. The increased use of fossil fuels has a harsh effect on the environment. Too much exploitation of fossil fuels increases the level of green house gas content in the atmosphere, resulting in global warming and a rise in the sea level.
It is not possible to completely reduce the consumption of fossil fuels. However, some measures can be taken such as using electrical appliances wisely and not wasting electricity. Unnecessary usage of water should be avoided. Public transport system with mass transit must be adopted on a large scale. These small steps may help in reducing the consumption of natural resources and conserving them.
Our Environment
Question 1:Why are some substances biodegradable and some non-biodegradable?
Answer
Some substances such as metal, glass, plastic, etc. which cannot be decomposed by the living organisms are non-biodegradable wastes. These substances are non-biodegradable because the micro-organisms do not have enzymes that can digest these substances. Therefore, we classify them as non-biodegradable wastes. Other substances such as paper, vegetable wastes, etc. that can be easily broken down by enzymes are biodegradable wastes.
Question 2: Give any two ways in which biodegradable substances would affect the environment.
Answer
Biodegradable substances affect the environment in the following ways:
(i) The biodegradable substances such as tree leaves, plant parts, and kitchen wastes can be used as humus after composting. This will enhance the soil fertility.
(ii) The biodegradable substances mainly contain carbon. These substances after decomposition release that carbon back into the atmosphere.
Question 3: Give any two ways in which non-biodegradable substances would affect the environment.
Answer
Non-biodegradable substances affect the environment in the following ways:
(i) They contaminate soil and water resources as they cannot be decomposed by micro-organisms.
(ii) These substances, when accidentally eaten by stray animals, can harm them and can even cause their death.
Question 4: What are trophic levels? Give an example of a food chain and state the different trophic levels in it.
Answer
A trophic level is the level of species in an ecosystem on the basis of the source of nutrition such as producers, primary consumers, secondary consumers, etc.
The producers form the first trophic level as they manufacture food. The primary consumers form the second trophic level, the secondary consumers form the third, and the tertiary consumers form the fourth trophic level.

Trophic levels:
Various trophic levels are connected through food chains. For example, in an aquatic food chain, phytoplanktons are the producers, zooplanktons are the primary consumers, and small fish is the secondary consumer and so on.

Quaternary consumer

Phytoplankton Tertiary consumer
Secondary consumer
Primary consumer
Primary producer
Aquatic food chain
Question 5: What is the role of decomposers in the ecosystem?
Answer
Decomposers include micro-organisms such as bacteria and fungi that obtain nutrients by breaking down the remains of dead plants and animals. They help in the breakdown of organic matter or biomass from the body of dead plants and animals into simple inorganic raw materials, such as
Question 6: What is ozone and how does it affect any ecosystem?
Answer
Ozone is a colourless gas that acts as a screen for ultraviolet radiation. It is continuously formed at the higher levels of the atmosphere due to the action of UV rays on molecular oxygen. The high energy UV radiations break down O2 molecules into nascent oxygen.
(Nascent oxygen)
Then, this free oxygen atom combines with an oxygen molecule to form ozone.
(Ozone)
In recent years, the amount of ozone in the atmosphere is getting depleted.
This ozone depletion causes a greater amount of ultra violet radiation to enter earth’s atmosphere. This has an indirect effect on the ecosystem.(Ecosystem includes both the biological community and the non-living components of an area). It results in the death of many phytoplanktons, thereby affecting the process of photosynthesis. Plants utilise atmospheric CO2 to make their food. In the absence of plants, the levels of
The depletion in the ozone layer also increases the frequency of infectious diseases as it suppresses the immune systems of both human beings and animals. The frequency of skin cancer also increases in human beings because of the direct exposure to ultraviolet radiations.
Question 7: How can you help in reducing the problem of waste disposal? Give any two methods.
Answer
The problem of waste disposal can be solved by proper waste management including the collection, transport, processing, and disposal of the waste materials.
The problem of waste management can be solved by the following given measures:
-Use separate bins (blue and green) for disposing non-biodegradable and biodegradable wastes.

Biodegradable waste

Different garbage bins for disposing biodegradable waste and non-biodegradable waste
Question 8: Which of the following groups contain only biodegradable items?
(a) Grass, flowers and leather
(b) Grass, wood and plastic
(c) Fruit-peels, cake and lime-juice
(d) Cake, wood and grass
Answer
(c)Fruit-peels, cake, and lime-juice
(d)Cake, wood, and grass
Question 9: Which of the following constitute a food-chain?
(a) Grass, wheat and mango
(b) Grass, goat and human
(c) Goat, cow and elephant
(d) Grass, fish and goat
Answer
(b) Grass, goat, and human
Question 10: Which of the following are environment-friendly practices?
(a) Carrying cloth-bags to put purchases in while shopping
(b) Switching off unnecessary lights and fans
(c) Walking to school instead of getting your mother to drop you on her scooter
(d) All of the above
Answer
(d) All of the above
Question 11: What will happen if we kill all the organisms in one trophic level?
Answer
Various trophic levels are connected through the food chains. If all the organisms of any one trophic level are killed, then it will disrupt the entire food chain.
For example, in a food chain, if all the plants are killed, then all the deer will die due to lack of food. If all the deer are dead, then soon the tigers will also die. Due to the death of these animals, the decomposer’s population will rise in that area.
This is just an example of one food chain. However, in nature, food chains are not isolated. They are interconnected in the form of food web. Therefore, killing all the plants of an area will not only affect the deer, it will also affect other herbivores such as goat, cattle, sheep, etc.
Question 12: Will the impact of removing all the organisms in a trophic level be different for different trophic levels? Can the organisms of any trophic level be removed without causing any damage to the ecosystem?
Answer
Organisms of all trophic levels are equally important and are an integral part of the ecosystem. If all the producers are removed, then it will affect all the herbivores as it is their primary food source. Death of herbivores will soon affect the primary carnivores and so on.
Now let us suppose that all the deer (herbivores) are killed in a region. This can lead to an increase in the number of producers. At the same time, there will be an increase in the number of other herbivores such as rabbits, goat, sheep, etc. due to less competition. This will also lead to the increase in population of only consumers of these increased herbivores. Thus, the balance in the ecosystem gets disturbed if any of its component organisms are removed.
Question 13: What is biological magnification? Will the levels of this magnification be different at different levels of the ecosystem?
Answer
Biomagnification is the increase in the concentration of pollutants or harmful chemicals within each step of the food chain. The levels of biomagnification will be different at different trophic levels. For example, in a pond of water, DDT was sprayed and the producers were found to have
DDT.

Biomagnifications
Question 14: What are the problems caused by the non-biodegradable wastes that we generate?
Answer
Non-biodegradable substances affect the environment in the following ways:
(i) Since the non-biodegradable substances cannot be broken down, they get accumulated and thus contaminate the soil and the water resources.
(ii) These substances, when accidentally eaten by some stray animal, can harm them and can even cause their death.
(iii) These substances occupy more space in the landfills and require special disposal techniques.
(iv) These materials can accumulate in the environment and can also enter the food chain.
Question 15: If all the waste we generate is biodegradable, will this have no impact on the environment?
Answer
Generation of only biodegradable waste will have a positive impact on the environment. There will not be any pollution caused by the non-biodegradable wastes. The problem associated with waste management and disposal will also not occur. The population of decomposers will increase to breakdown the extra biodegradable waste generated.
Question 16: Why is damage to the ozone layer a cause for concern? What steps are being taken to limit this damage?
Answer
Ozone depletion occurs widely in the stratosphere. However, it is more prominent over the Antarctic region and is known as the ozone hole.

Diagram representing ozone hole
Consequences of ozone depletion:
- It causes skin darkening, skin cancer, ageing, and corneal cataracts in human beings.
- It can result in the death of many phytoplankton’s that leads to increased global warming.
To limit the damage to the ozone layer, the release of CFCs into the atmosphere must be reduced. CFCs used as refrigerants and in fire extinguishers should be replaced with environmentally-safe alternatives. Also, the release of CFCs through industrial activities should be controlled.
Management of Natural Resources
Question 1: What changes can you make in your habits to become more environment-friendly?
Answer
We should switch off the electrical appliances when not in use. Water and food should not be wasted. Close the tap when not in use. Dump the objects made of plastic and glass in designated recycling boxes. Plastic, paper, or glass must be recycled or reused and not dumped with other wastes. This is because objects made of plastic do not get decomposed easily. Besides soil fertility, they badly affect our environment. We should dispose the wastes safely and not disperse in public places. These are a few things that can be done to become more environmentfriendly.
Question 2: What would be the advantages of exploiting resources with short-term aims?
Answer
There should be a judicious use of natural resources as they are limited in nature. We should not exploit resources for our short term gains as this would only lead to depletion of natural resources for the present generation as well as generations to come. Hence, we can say that there are hardly any advantages of exploiting natural resources for short term gains.
Question 3: How would these advantages differ from the advantages of using a long-term perspective in managing our resources?
Answer
In the case of a long-time perspective in managing our resources, these resources will last for the generations to come. This management ensures uniform distribution among the people. It conserves the natural resources for many years and not just for a few years, as in the case of a shortterm perspective in conserving natural resources.
Question 4: Why do you think there should be equitable distribution of resources? What forces would be working against an equitable distribution of our resources?
Answer
Natural resources of the Earth must be distributed among the people uniformly so that each and every one gets his share of the resource.
Human greed, corruption, and the lobby of the rich and powerful are the forces working against an equitable distribution of resources.
Question 5: Why should we conserve forests and wildlife?
Answer
We should conserve forests and wildlife to preserve the biodiversity (range of different life-forms) so as to avoid the loss of ecological stability. A large number of tribes are the habitants in and around the forests. If the forests are not conserved, then it may affect these habitants.
Without proper management of forest and wildlife, the quality of soil, the water sources, and even the amount of rainfall may be affected. Without forest and wildlife, life would become impossible for human beings.
Question 6: Suggest some approaches towards the conservation of forests.
Answer
Some approaches towards the conservation of forests are as follows:
(a) People should show their participation in saving the forest by protesting against the cutting of trees. For example, Chipko Andolan
(b) Planting of trees should be increased. Rate of afforestation must be more than that of deforestation.
(c) Some people cut precious trees such as Chandan to earn money. Government should take legal steps to catch these wood smugglers.
(d) Habitants of forests must not be bothered by the forest officials. Otherwise, this would result in the clash between tribal people and the government officials, thereby enhancing the naxal activities in forests
Question 7: Find out about the traditional systems of water harvesting/management in your region.
Answer
One of the traditional systems of water harvesting used in our region is tanks.
Question 8: Compare the above system with the probable systems in hilly/mountainous areas or plains or plateau regions.
In plains, the water harvesting structures are crescent-shaped earthen embankments. These are low, straight, and concrete.
In hilly regions, the system of canal irrigation called Kulhs is used for water harvesting. This involves a collection of rain water in a stream, which is then diverted into man-made channels down the hill sides
Question 9: Find out the source of water in your region/locality. Is water from this source available to all people living in that area?
Answer
The source of water in our region is ground water. Water from the source is available to all the people living in that area.
Question 10: What changes would you suggest in your home in order to be environment-friendly?
Answer
Changes that can be undertaken in our homes to be environment-friendly are listed below:
(i)Switch off the electrical appliances when not in use.
(ii)Turn the taps off while brushing or bathing and repair the leaking taps.
(iii)Throw biodegradable and non-biodegradable waste into separate bins.
(iv)Construct composting pits.
(v)Food items such as jam, pickles, etc., come packed in plastic bottles. These bottles can later be used for storing things in the kitchen.
Question 11: Can you suggest some changes in your school which would make it environmentfriendly?
Answer
Changes that can be undertaken in our schools to make it environment friendly are listed below:
(i) Electricity can be saved by switching off lights and fans when not required.
(ii) Turn the taps off when not in use.
(iii) Biodegradable and non-biodegradable wastes should be thrown into separate bins.
Question 12: We saw in this chapter that there are four main stakeholders when it comes to forests and wildlife. Which among these should have the authority to decide the management of forest produce? Why do you think so?
Answer
The forest department of the government should have the authority to decide the management of forest produces. This is because the forest department is the care taker of the forest land and is responsible for any damage to the forest.
Question 13: How can you as an individual contribute or make a difference to the management of (a) forests and wildlife, (b) water resources and (c) coal and petroleum?
Answer
(a) Forest and wildlife:
(i) We should protest against the cutting of trees (deforestation).
(ii) We should protest against the poaching of wild animals.
(iii) We should stop the annexation of forest land for our use.
(b) Water resources:
(i) Turn the taps off while brushing or bathing and repair leaking taps.
(ii) We should practice rainwater harvesting.
(iii) We should avoid the discharge of sewage and other wastes into rivers and other water resources.
(c) Coal and petroleum:
(i) We should take a bus or practice car pooling to avoid excessive use of petroleum.
(ii) We should stop using coal as a fuel (angithis).
(iii)We should use alternative sources of energy such as hydro-energy and solar energy instead of depending largely on coal and petroleum.
Question 14: What can you as an individual do to reduce your consumption of the various natural resources?
Answer
Natural resources such as water, forests, coal and petroleum, etc. are important for the survival of human beings. The ways in which we can reduce the consumption of various natural resources are as follows:
(i) We should stop the cutting of trees (deforestation).
(ii) We should use recycled paper to reduce the cutting down of trees.
(iii) We should not waste water.
(iv) We should practice rainwater harvesting.
(v) We should practice car pooling to avoid the excessive use of petroleum.
(vi) We should use alternative sources of energy such as hydro-energy and solar energy.
Question 15: List five things you have done over the last one week to -
(a) conserve our natural resources
(b) increase the pressure on our natural resources
Answer
(a) To conserve our natural resources:
(i) Travel by a CNG bus for long distances and walk for short distances.
(ii) Use recycled paper
(iii) Throw biodegradable and non-biodegradable waste into separate bins
(iv) Plant trees
(v) Harvest rainwater
(b) To increase the pressure on our natural resources:
(i) Use non-renewable resources of energy
(ii) Waste water
(iii) Waste electricity
(iv) Use plastics and polythene bags for carrying goods
(v) Use escalators
Question 16: On the basis of the issues raised in this chapter, what changes would you incorporate in your life-style in a move towards a sustainable use of our resources?
Answer
One should incorporate the following changes in life-style in a move towards a sustainable use of our resources:
(i) Stop cutting trees and practice plantation of trees.
(ii) Stop using plastic and polythene bags for carrying goods. (iii) Use recycled paper.
(iv) Throw biodegradable and non-biodegradable waste into separate bins.
(v) Waste minimum amount of water while using and repair leaking taps.
(vi) Practice rainwater harvesting.
(vi) Avoid using vehicles for short distances. Instead, one can walk or cycle to cover short distances. To cover long distances, one should take a bus instead of using personal vehicles.
(vii) Switch off electrical appliances when not in use.
(viii) Use fluorescent tubes in place of bulbs to save electricity.
(ix) Take stairs and avoid using lifts.
(x) During winters, wear an extra sweater to avoid using heaters.






