Unit 11 Alcohols, Phenols And Ethers (Intext Questions-5)
Intext Questions
11.10 Write the reactions of Williamson synthesis of 2-ethoxy-3-methylpentane starting from ethanol and 3-methylpentan-2-ol.
Show Answer
Answer
In Williamson synthesis, an alkyl halide reacts with an alkoxide ion. Also, it is an $\mathrm{S_\mathrm{N}} 2$ reaction. In the reaction, alkyl halides should be primary having the least steric hindrance. Hence, an alkyl halide is obtained from ethanol and alkoxide ion from 3-methylpentan-2-ol.
$ \underset{\text{Ethanol}}{\mathrm{C_2} \mathrm{H_5} \mathrm{OH}} \xrightarrow{\mathrm{HBr}} \underset{\text{Bromoethane}}{\mathrm{C_2} \mathrm{H_5} \mathrm{Br}} $

11.11 Which of the following is an appropriate set of reactants for the preparation of 1-methoxy-4-nitrobenzene and why?

Show Answer
Answer
Set (ii) is an appropriate set of reactants for the preparation of 1-methoxy-4-nitrobenzene.

1-Methoxy - 4- nitrobenzene
In set (i), sodium methoxide $\left(\mathrm{CH_3} \mathrm{ONa}\right)$ is a strong nucleophile as well as a strong base. Hence, an elimination reaction predominates over a substitution reaction.
11.12 Predict the products of the following reactions:
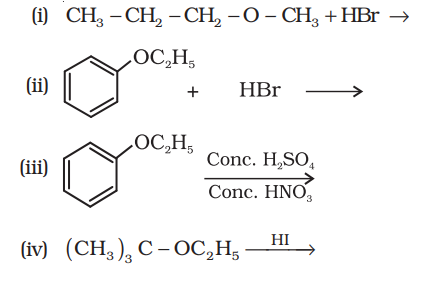
Show Answer
Answer
(i)
$ \underset{n-\text{propylmetyl ether}}{\mathrm{CH_3}-\mathrm{CH_2}-\mathrm{CH_2}-\mathrm{O}-\mathrm{CH_3}}+\mathrm{HBr} \longrightarrow \underset{\text{Propanol}}{\mathrm{CH_3}-\mathrm{CH_2}-\mathrm{CH_2}-\mathrm{OH}}+ \underset{\text{Bromomethane}}{\mathrm{CH_3}-\mathrm{Br}}$
(ii)

(iii)

(iv)
$$ \underset{\text { tert -Butyl ethyl ether }}{\left(\mathrm{CH_3}\right) _{3} \mathrm{C}-\mathrm{OC _2} \mathrm{H_5}} \xrightarrow{\mathrm{HI}} \underset{\text { tert } \text {-Butyliodide }}{\left(\mathrm{CH _3}\right) _{3} \mathrm{C}-\mathrm{I}}+\underset{\text{Ethanol}}{\mathrm{C _2} \mathrm{H _5} \mathrm{OH}} $$










