Chapter 04 Carbon and its Compounds Exercise
EXERCISES
1. Ethane, with the molecular formula $C_2 H_6$ has
(a) 6 covalent bonds.
(b) 7 covalent bonds.
(c) 8 covalent bonds.
(d) 9 covalent bonds.
Show Answer
Answer
(b) Ethane has 7 covalent bonds.
2. Butanone is a four-carbon compound with the functional group
(a) carboxylic acid.
(b) aldehyde.
(c) ketone.
(d) alcohol.
Show Answer
Answer
(c) The functional group of butanone is ketone.
3. While cooking, if the bottom of the vessel is getting blackened on the outside, it means that
(a) the food is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the fuel is burning completely.
Show Answer
Answer
(b) While cooking, if the bottom of the vessel is getting blackened on the outside, then it means that the fuel is not burning completely.
4. Explain the nature of the covalent bond using the bond formation in $CH_3 Cl$.
Show Answer
Answer
Carbon can neither lose four of its electrons nor gain four electrons as both the processes require extra amount of energy and would make the system unstable. Therefore, it completes its octet by sharing its four electrons with other carbon atoms or with atoms of other elements. The bonds that are formed by sharing electrons are known as covalent bonds. In covalent bonding, both the atoms share the valence electrons, i.e., the shared electrons belong to the valence shells of both the atoms.

Here, carbon requires 4 electrons to complete its octet, while each hydrogen atom requires one electron to complete its duplet. Also, chlorine requires an electron to complete the octet. Therefore, all of these share the electrons and as a result, carbon forms 3 bonds with hydrogen and one with chlorine.
5. Draw the electron dot structures for
(a) ethanoic acid.
(b) $H_2 S$.
(c) propanone.
(d) $F_2$.
Show Answer
Answer
(a) Ethanoic acid
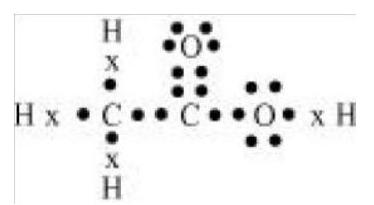
(b) H2 S

(c) Propanone

(d) F2
6. What is an homologous series? Explain with an example.
Show Answer
Answer
A homologous series is a series of carbon compounds that have different numbers of carbon atoms but contain the same functional group.
For example, methane, ethane, propane, butane, etc. are all part of the alkane homologous series. The general formula of this series is $CnH 2 n+2$.
Methane $CH_4$
Ethane $CH_3CH_3$
Propane $CH_3CH_2 CH_3$
Butane $CH_3 CH_2 CH_2 C_3$
It can be noticed that there is a difference of - $CH_2$ unit between each successive compound.
7. How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties?
Show Answer
Answer
- Ethanol is a liquid at room temperature with a pleasant odour while ethanoic acid has vinegar-like smell. The melting point of ethanoic acid is $17^{\circ} C$. This is below room temperature and hence, it freezes during winters.
- Ethanoic acid reacts with metal carbonates and metal hydrogencarbonates to form salt, water, and carbon dioxide gas while ethanol does not react with them.
Metal Carbonates/Metal Hydrogencarbonates + Carboxylic acid $\longrightarrow \text{ Salt }+ \text{ Water }+ \text{ Carbon dioxide } $
For example,
$ 2 CH_3 COOH+Na_2 CO_3 \longrightarrow 2 CH_3 COONa+H_2 O+CO_2 $
Metal Carbonates/Metal Hydrogencarbonates + Alcohols $\longrightarrow$ No reaction
For example,
$ CH_3 CH_2 OH+Na_2 CO_3 \longrightarrow \text{ No reaction } $
8. Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
Show Answer
Answer
A soap is a sodium or potassium salt of long chain fatty acids. It has one polar end and one non-polar end. The polar end is hydrophilic in nature i.e., this end is attracted towards water. The non-polar end is hydrophobic but lipophilic, i.e., it is attracted towards hydrocarbons. When soap is added to water, soap molecules arrange themselves in a cluster to keep the non-polar portion out of water such that the non-polar ends are in the interior of the cluster and the polar ends are on the surface of the cluster. Since the dirt present on clothes is organic in nature and insoluble in water, the hydrophobic ends of the clusters attach themselves to the dirt. This cluster formation in which the dirt is entrapped is the micelle.

Micelle formation does not occur in alcohol because the alkyl chain of soap becomes soluble in alcohol.
9. Why are carbon and its compounds used as fuels for most applications?
Show Answer
Answer
Most of the carbon compounds give a lot of heat and light when burnt in air. Saturated hydrocarbons burn with a clean flame and no smoke is produced. The carbon compounds, used as a fuel, have high calorific values. Therefore, carbon and its compounds are used as fuels for most applications.
10. Explain the formation of scum when hard water is treated with soap.
Show Answer
Answer
Soap does not work properly when the water is hard. A soap is a sodium or potassium salt of long chain fatty acids. Hard water contains salts of calcium and magnesium. When soap is added to hard water, calcium and magnesium ions present in water displace sodium or potassium ions from the soap molecules forming an insoluble substance called scum. A lot of soap is wasted in the process.
11. What change will you observe if you test soap with litmus paper (red and blue)?
Show Answer
Answer
Since soap is basic in nature, it will turn red litmus blue. However, the colour of blue litmus will remain blue.
12. What is hydrogenation? What is its industrial application?
Show Answer
Answer
Hydrogenation is the process of addition of hydrogen. Unsaturated hydrocarbons are added with hydrogen in the presence of palladium and nickel catalysts to give saturated hydrocarbons.
This reaction is applied in the hydrogenation of vegetables oils, which contain long chains of unsaturated carbons.
13. Which of the following hydrocarbons undergo addition reactions: $C_2 H_6, C_3 H_8, C_3 H_6, C_2 H_2$ and $CH_4$.
Show Answer
Answer
Unsaturated hydrocarbons undergo addition reactions. Being unsaturated hydrocarbons, $C_3H_6$and $C_2H_2$ undergo addition reactions.
14. Give a test that can be used to differentiate between saturated and unsaturated hydrocarbons.
Show Answer
Answer
Butter contains saturated fats. Therefore, it cannot be hydrogenated. On the other hand, oil has unsaturated fats. That is why it can be hydrogenated to saturated fats (solids).
15. Explain the mechanism of the cleaning action of soaps.
Show Answer
Answer
Cleansing action of soaps:
The dirt present on clothes is organic in nature and insoluble in water. Therefore, it cannot be removed by only washing with water. When soap is dissolved in water, its hydrophobic ends attach themselves to the dirt and remove it from the cloth. Then, the molecules of soap arrange themselves in micelle formation and trap the dirt at the centre of the cluster. These micelles remain suspended in the water. Hence, the dust particles are easily rinsed away by water.











