Chapter 09 Modern Physics
Introduction
All matters are composed of what is called ‘atom’. Atom is composed of fundamental particles and it has a nucleus in which its mass is concentrated.
The story of how atom was discovered is very long. But the brief story is that first of all Thomson provided something acceptable in scientific community about the atom. After him, Rutherford presented the modified and almost acceptable atomic model but it had also some discrepencies which was removed by Neils Bohr when he presented the most updated form of the atomic model in 1913.
This chapter provides us the structure of atom and its different features. The chapter also gives a glimpse of the nucleus and different phenomena occuring in it like radioactivity, fission, fusion etc.
You will also study the applications of these nuclear phenomena such as in atom bomb, hydrogen bomb, nuclear reactor and so on.
ATOMIC MODEL THOMSON ATOMIC MODEL
This model suggests an atom to be a tiny sphere of radius
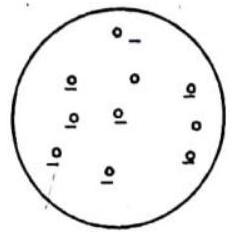
Fig 9.1 Thomson’s atomic model
This model failed to explain (i) large scattering angle of
RUTHERFORD’S ATOMIC MODEL
(a) Atom is sphere of diameter about
(b) The diameter of the nucleus is of the order of
(c) the space around the nucleus is almost empty. It is filled only with tiny sized electrons which revolve around the nucleus in different orbits.
(d) The electrostatic attraction between the nucleus and electron provides centripetal force required for circular motion
(e) Total positive charge in the nucleus is equal to total -ve charge at the atom.
Drawback of Rutherford Model
(1) The electron orbiting around the nucleus is under acceleration. So it must radiate energy. This may result in radius of orbits decreasing gradually.
(2) According to the Rutherford electron can revolve in all possible orbits hence the atom should emit radiation of all possible wave length. But in reality atoms are found to have line spectrum.
BOHR’S ATOMIC MODEL
In 1913 Bohr gave his atomic theory primarily to explain, the spectra of hydrogen & hydrogen-like atoms. His theory, contained a combination of views from Plank’s quantum theory, Einstein’s photon concept & Rutherford model of atom. The Bohr theory can explain, the atomic spectra of hydrogen atom & hydrogen-like ions such as
(i) The electron moves in circular orbits around the nucleus under the influence of coulombic force of attraction between the electron and the positively charged nucleus (as shown in figure below).
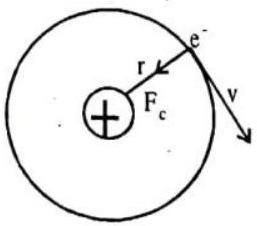
Fig.9.2 Bohr’s model of hydrogen atom
(ii) The electron rotates about the nucleus in certain stationary circular orbits, for which the angular momentum of electron about the nucleus is an integral multiple of
(iii) When the electron is in one of its stationary orbits, it does not radiate energy, hence the atom is stable.These stationary orbits are called allowed orbits.
(iv) The atom radiates energy when the electron “jumps” from one allowed stationary state to another. The frequency of radiation follows the condition
Where
Now, we calculate the allowed energies of hydrogen atom, by using the model shown in fig (i), in which the electron travels in a circular orbital of radius
For moving an electron in a circular orbit the required centripetal force is provided by the coulomb force of attraction which acts between nucleus
where
Eliminating
(radius of
Equation (4) gives the radii of various orbits (have discrete values).
The smallest radius (also called Bohr radius) corresponds to
From equations (4) & (1) we obtain,
or
(velocity in nth state)
The total energy of electron is given by
(Allowed energy state)
After substituting numerical values in Eq.(7), we obtain
KEEP IN MEMORY
1. Total energy
The second line of lyman series is when electron jumps from
The limiting line of lyman series is when electron jumps from
The lowest energy state, or ground state, corresponds to
The next state corresponds to
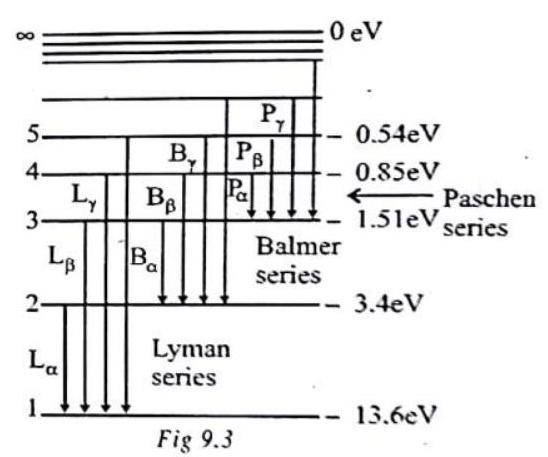
An energy level diagram of the hydrogen atom is shown in figure 2. The upper most level corresponding to
Energy level diagram for hydrogen atom
Some transitions for Layman, Balmer & Paschen series are shown. The quantum numbers are at left & energies of levels are at right.
In this case ……
If the electron jumps from allowed state
and the wavelength of emitted photon is
and
Where
(i) Layman series
(iii) Paschen series
(v)
(ii) Balmer series
(iv) Brackett series
First three series of hydrogen atom are shown in figure. But in practice, the value of Rydberg constant varies between
This is because in above calculations we assumed that electron revolves around a massive fixed nucleus of mass
So total energy by taking this correction is
If we are dealing with hydrogen like ions such as
and the allowed energies are given by
WAVELENGTH LIMITS IN VARIOUS SPECTRAL SERIES OF HYDROGEN ATOM
(i) For Lyman series (in ultraviolet region)
Here
(ii) For Balmer Series (in visible region)
Here
(iii) For Paschen series (in infrared region)
Here
(iv) For Brackett series (in infrared region)
Here
(v) For p-fund series (in infrared region)
Here
KNOWLEDGE ENHANCER
If Bohr’s quantization postulate (angular momentum
Angular momentum
For such large value of
LIMITATIONS OF BOHR’S MODEL
1. It could not explain the spectra of atoms containing more than one electron.
2. There was no theoretical basis for selecting
KEEP IN MEMORY
1. Energy of electrons in different orbits in an atom varies inversely with the square of the number of orbits. So, energy of electrons increases (decreases in negative) as the orbit becomes higher.
2. If energy of a particular orbit is
KNOWLEDGE ENHANCER
An electron drops from the fourth energy level in an atom to the third level and then to the first level. Two frequencies of light are emitted. How does their combined energy compare with the energy of the single frequency that would have been emitted if the electron had dropped from the fourth level directly to the first level?
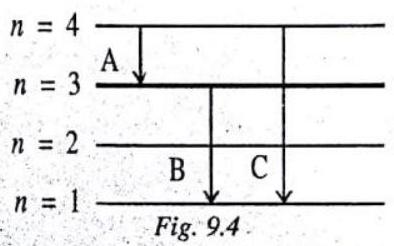
In this figure we have shown the drop of an electron from fourth energy level
ILLUSTRATION-9.1
Ionization potential of hydrogen atom is
Show Answer
SOLUTION:
Given that
After absorbing
This energy corresponds to
Now following three transitions are possible
ILLUSTRATION-9.2
The ionisation energy of Hydrogen atom is 13.6 eV. Following Bohr’s theory, what is the energy corresponding to a transition between the 3rd and the 4th orbit?
Show Answer
SOLUTION:
ILLUSTRATION-9.3
If the wavelength of the first line of the Lyman series for the hydrogen atom is
Show Answer
SOLUTION:
We know that,
For first line of Lyman series,
for first line of Balmer series
or,
ILLUSTRATION-9.4
If the electron in hydrogen atom jumps from the third orbit to second orbit, what is the wavelength of the emitted radiation?
Show Answer
SOLUTION:
DISCHARGE TUBE EXPERIMENT
When a very strong potential difference is applied across the two electrodes in a discharge tube and the preacure of the sir is lowered gradually, then a stage is reached at which the current begins to flow through the air with cracking noise. The potential at which this happens is called sparking potential.
As pressure is lowered to
At
The luminous streaks travelling from cathode to anode, below
Fig. 9.5
PROPERTIES OF CATHODE RAYS
Emitted perpendicularly to cathode, travel in straight lines, carry energy, possess momentum, deflected by electric and magnetic fields, excite fluroescence, ionise gas, produce highly penetrating secondary radiation when suddenly stopped, effect photographic plate.
POSITIVE RAYS
Stream of +ve ions moving towards cathode in discharge tube.
PROPERTIES OF POSITIVE RAYS
(i) Rays are made up of positively charged particles.
(ii) Being heavy, their velocity is much less than that of cathode rays.
(iii) They ionise the gas through which they pass and ionising power of positive rays is more than that of cathode rays.
(iv) +ve rays are deflected by electric & magnetic field showing that they carry +ve charge with them.
PHOTO ELECTRIC EFFECT
In
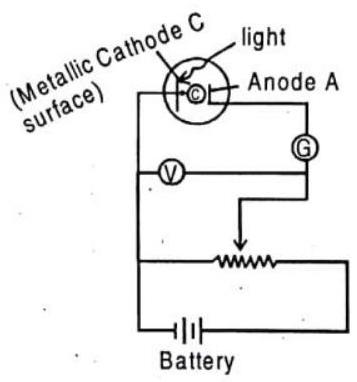
Fig. 9.6 When light strikes the cathode
Figure shows, when light strikes the cathode
A plot of photoelectric current versus the potential difference
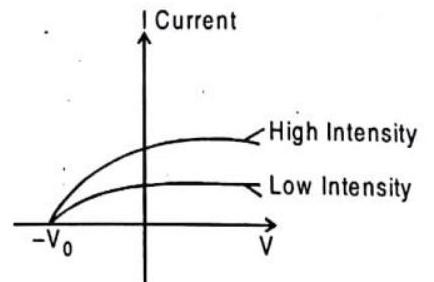
Fig. 9.7 Photoelectric current versus voltage for two light intensities. The current increases with intensity, but reaches a saturation. At voltage less than
It is clear from figure that photo electric current increases as we increase the intensity of light & obtain saturation value at larger value of potential difference
Maximum Kinetic energy of electron
or
where
But some features of photo electric effect can not be explained by classical physics & the wave theory of light.
(1) No photo electrons are emitted, if the frequency of incident light is less than some cut-off frequency (i.e., threshold frequency)
(2) The maximum kinetic energy of the photo electrons is independent of light intensiy, but increases with increasing the frequency of incident light.
(3) Electrons are emitted from surface almost instantaneously (less than
These above points were explained by Einstein in 1905 by treating the light as stream of particles. Taking Max Planck assumption, Einstein postulated that a beam of light consists of small packets of energy called photons or quanta. The energy
Where
When a photon arrives at surface, it is absorbed by an electron. This energy transfer is an All-or-Nothing process, in contrast to continuous transfer of energy in classical theory; the electrons get all photon’s energy or none at all. If this energy is greater than the work function
To obtain maximum kinetic energy
Where
VARIOUS GRAPHS RELATED TO PHOTO ELECTRIC EFFECT
Fig. 9.8
WORK FUNCTIONS OF SOME ELEMENTS :
| Element | (Work function) (eV) |
|---|---|
Where
Within the frame work of photon theory of light (Quantum theory of light) we can explain above failures of classical physics.
(1) It is clear from eq. (3) that if energy of photon is less than the work function of metallic surface, the electrons will never be ejected from surface regardless of intensity of incident light.
(2)
(3) Electrons are emitted almost instantaneously consistent with particle view of light in which incident energy is concentrated in small packets (called photons) rather than over a large area (as in wave theory).
Knowledge ENHANCER
Every metal has a definite work function. Why do all photoelectrons not come out with the same energy if incident radiation is monochromatic? Why is there an energy distribution of photoelectrons?
By work function of a metal, we mean the minimum energy required for the electron in the highest level of conduction band to get out of the metal. Since all the electrons in the metal do not belong to that level but they occupy a continuous band of levels, therefore, for the given incident radiation, electrons knocked off from different levels come out with different energies.
X-RAYS
The X-rays were discovered by Prof. Roentgen, a German scientist in 1885. He was awarded Nobel Prize for this discovery in1901.
The modern apparatus for the production of X-rays was developed by Dr. Coolidge in 1913.
X-rays are produced when fast moving electrons are suddenly stopped on a metal of high atomic number.
Production of X-rays
The modern device used in the production of X-ray is called the Coolidge tube, which consists of a highly evacuated glass tube as shown in the figure.
A filament
The fast moving electrons produce X-rays when they strike the target.
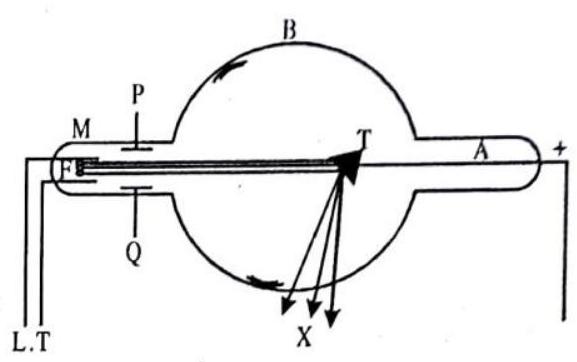
Fig. 9.9 Coolidge tube
Application of
1. Scientific Applications:
The diffraction of
2. Industrial Applications:
Since
3. In Radio Therapy:
X-rays can cause damage to the tissues of body (cells are ionized and molecules are broken). So X-rays damages the malignant growths like cancer and tumors which are dangerous to life, when it used in proper and controlled intensities.
4. In Medicine and Surgery:
ILLUSTRATION-9.5
The work function of cesium is
Show Answer
SOLUTION:
(a)
Threshold wavelength
(b)
(c)
(d) The K.E. of emitted electrons does not depend upon the intensity of light. Hence if the intensity of incident light be doubled, the energy will remain unchanged.
NUCLEAR FISSION
An atom’s nucleus can be split apart. When this is done, a tremendous amount of energy is released. The energy is both heat and light energy. Einstein said that a very small amount of matter contains a very large amount of energy. This energy, when let out slowly, can be harnessed to generate electricity. When it is let out all at once, it can make a tremendous explosion in an atomic bomb. The word fission means to split apart. Inside the reactor of an atomic power plant, uranium atoms are split apart in a controlled chain reaction.
The shape of nucleus is distorted and nucleus splits into two fragments emitting several neutrons.
Fig. 9.10
The energy released in fission of Uranium is about
In a chain reaction, particles released by the splitting of the atom go off and strike other uranium atoms splitting those. Those particles given off split still other atoms in a chain reaction. In nuclear power plants, control rods are used to keep the splitting regulated so it doesn’t go too fast.
If the reaction is not controlled, you could have an atomic bomb. But in atomic bombs, almost pure pieces of the element Uranium235 or Plutonium, of a precise mass and shape, must be brought together and held together, with great force. These conditions are not present in a nuclear reactor.
The reaction also creates radioactive material. This material could hurt people if released, so it is kept in a solid form. The very strong concrete dome is designed to keep this material inside if an accident happens.
Energy released per gm of Uranium
Energy released by
This energy is equivalent to
(i) energy obtained by burning
(ii) energy obtained by burning 20 tonne of explosive TNT
The energy is released in form of kinetic energy of fission fragments,
The fission process can take place at normal pressure and temperature.
NUCLEAR FUSION
Another form of nuclear energy is called fusion. Fusion means joining smaller nuclei to make a larger nucleus. The sun uses energy from nuclear fusion of hydrogen atoms into helium atoms. This gives off heat and light and other radiation.
Also given off in this fusion reaction is energy.
Scientists have been working on controlling nuclear fusion for a long time, trying to make a fusion reactor to produce electricity. But they have been having trouble learning how to control the reaction in a contained space.
Controlled nuclear fusion is believed by many scientists to be the ultimate solution to the world’s energy problems. The energy released in fusion reactions is many times greater than that released in fission reactions. To date, however, the technology has not been developed to make use of this source of energy. What’s better about nuclear fusion is that it creates less radioactive material than fission, and its supply of fuel can last longer than the sun’s.
Fusion is possible at high pressure
The proton-proton cycle happens at lower temperature as compared to carbon-nitrogen cycle.
Nuclear fusion in possible at a place which has reactants in large quantity.
Hydrogen bomb works on principle of nuclear fusion.
The explosion of a hydrogen bomb needs an explosion of atom bomb to generate required temperature.
No harmful radiations are produced in fusion.
COMPARISON OF FISSION AND FUSION
| Nuclear fission | Nuclear fusion |
|---|---|
| 1. A heavy nucleus splits into two lighter nuclei. | 1. Two lighter nuclei join to form a heavy nucleus. |
| 2. Neutrons are required to start fission. | 2. Protons are usually required to start fusion. |
| 3. It takes place at normal pressure and temperature. | 3. It takes place at very high pressure and temperature. |
| 4. The energy released per nucleon is small |
4. The energy released per nucleon is large |
| 5. Fissionable material is expensive and not easily available. | 5. Fusion material is cheap and abunduntly available. |
| 6. Energy released in fission per cycle is large |
6. Energy released in fusion per cycle is small |
NUCLEAR REACTORS
A peaceful application of nuclear fission is the generation of electricity using heat from a controlled chain reaction in a nuclear reactor. A nuclear reactor is an arrangement in which the energy produced (in the form of heat) in a nuclear fission can be used in a controlled manner to produce steam, which can run the turbine and produce electricity.
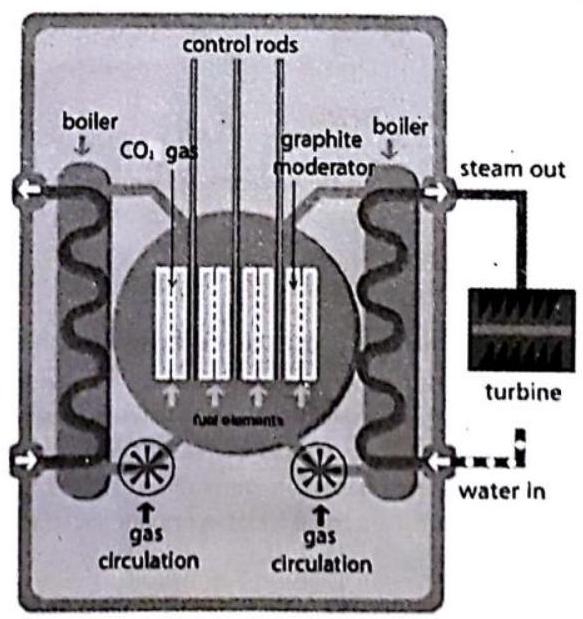
Fig. 9.11 : Nuclear Reactor
The main part of nuclear reactor
(a) Nuclear fuel:
It is the fissionable material used in nuclear reactors to produce energy by fission process. The nuclear fuel consists of uranium, usually in the form of its oxide,
(b) Moderator:
An important aspect of the fission process is the speed of the neutrons. Slow neutrons hit uranium-235 nuclei more efficiently than do the fast ones. Because fission reactors are highly exothermic, the neutrons produced usually move with high velocities. For greater collision efficiency, neutrons must be slowed down. For this purposes a substance is used that can reduce the kinetic energy of neutrons. Such a substance is called as a moderator. A good moderator should be a nontoxic and inexpensive substance. And it should resist conversion into radioactive substance by neutron bombardment. Graphite (C) or heavy water
(c) Control rods:
In principle, the main difference between an atomic bomb and nuclear reactor is that the chain reaction that takes place in a nuclear reactor is kept under controlled conditions at all the times. The factor limiting the rate of the reaction is the number of neutrons present. This can be controlled by lowering cadmium or boron rods between the fuel elements.
(d) Coolant:
It is the substance which is circulated in pipes to absorb the heat given off by the nuclear reactor and transfer it outside the reactor core, where it is used to produce steam to drive an electric generator. Large quantity of water is used as coolant.
(e) Shield:
To prevent the losses of heat and to protect the people operating the reactor from the radiation and heat, the entire reactor core is enclosed in a heavy steel or concrete dome, called the shield.
A complete nuclear power plant essentially consists of the four parts: reactor core, steam generator, steam turbine, and steam condensing system.
ABOUT THE NUCLEUS
Rutherford proposed the existence of a nucleus in 1911 to explain the results of his
Neutron
Neutron is a neutral particle carrying no charge
(i) mass of neutron
(ii) they are not deflected by external electric and magnetic fields
(iii) neutrons have high penetrating power and low ionizing power
(iv) neutrons are stable inside the nucleus. Outside the nucleus they are unstable with a half life of about 13 minutes
(v) neutron was discovered by James Chadwick in 1932 when he tried to explain results of collision of
(vi) The spin angular momentum of a neutron is
(vii) depending on speed they are classified as fast and slow (thermal) neutrons.
Proton
Proton is a charged particle carrying unit positive charge.
(i) mass of proton
(ii) proton was discovered by Goldstein in 1919.
(iii) The number of protons present inside the nucleus of an atom is called atomic number (
(iv) As atom is electrically neutral so number of protons inside the nucleus is equal to number of electrons in an atom.
According to Heisenberg a proton and neutron can be regarded as two different charge states of same particle called nucleon. The total number of protons and neutrons present inside the nucleus is known as mass number
A nuclide is a specific nucleus of an atom characterized as
TYPES OF NUCLEI
Isotopes :
These are nuclei of same element having same
e.g.
All isotopes of an element have same chemical properties. They occupy same place in periodic table. They cannot be separated by chemical analysis. They can be separated by mass spectrometers or mass spectrographs.
Isotones
These are nuclei of different elements having same
e.g. .
Isotones are different elements with different chemical properties. They occupy different positions in periodic table. They can be separated by chemical analysis and mass spectrometers.
Isobars
These are nuclei of different elements having same
e.g
Isobars are different elements with different chemical properties. They occupy different positions in periodic table. They can be separated by chemical analysis but cannot be separated by mass spectrometers.
Mirror nuclei
These are nuclei with same
Isomer nuclei
These are nuclei with same
NUCLEAR FORCES
The strong forces of attraction which firmly hold the nucleons in the small nucleus and account for stability of nucleus are called as nuclear forces.
The nuclear force is a short range force
(i) They are appreciable when distance between nucleons is of the order of
(ii) They become negligible when distance between nucleons is greater than
(iii) When distance between two nucleons is less than
Nuclear forces are charge independent
(a) force between a pair of protons, a pair of neutrons and a pair of neutron and proton is equal.
(b) The net force between pair of neutrons and a pair of neutron and proton is equal. This is slightly greater than force between pair of protons because force between protons is reduced due to electrostatic repulsion Net force
Nuclear forces are spin dependent
(a) Nuclear force depends on relative orientation of spins between two interacting nucleons. (b) The force of attraction between two nucleons with parallel spin is greater than force between nucleons with antiparallel spin.
(c) Deutron is formed in a bound state only if spins of neutron and proton are parallel.
Nuclear forces show saturation property
(a) The nucleon in nucleus interacts with its nearest neighbour only.
(b) It remains unaffected by the presence of other surrounding nucleons.
(c) The nuclear force between a pair of nucleons in light and heavy nucleus is equal.
Nuclear forces are non-central forces
(a) They do not act along line joining the centre of two nucleons.
(b) The non-central component depends on orientation of spins relative to line joining the centre of two nucleons.
Size of nucleus :
Rutherford in his
When
Rutherford assumed the distance of closest approach as a measure of size of nucleus.
Radius of nucleus is related to mass number as
Density of nucleus :
Volume of nucleus
mass of nucleus
density of nucleus
The nuclear density is independent of mass number
The nuclear density is nearly constant and is equal to
The nuclear density is maximum at centre of nucleus and decreases as one moves away from the centre.
The distance from the centre of nucleus where density becomes
The high density of nucleus indicates compactness of nucleus.
Atomic mass unit :
1 atomic mass unit
Energy equivalent to 1 amu mass,
MASS DEFECT
The mass of the nucleus is always less than the sum of masses of nucleons composing the nucleus. The difference between the
rest mass of nucleus and sum of rest masses of nucleons constituting the nucleus is known as mass defect.
Mass defect
BINDING ENERGY
The energy required to break a nucleus into its constituent nucleons and place them at infinite distance is called binding energy
The energy equivalent to mass defect is called binding energy. This is the energy with which the nucleons are held together.
The binding energies
Binding energy
rest mass of protons + rest mass of neutrons
NUCLEAR REACTION
The transformation of one stable nucleus into another nucleus by bombardment with suitable high energy particles like proton,neutron,
e.g.
The nuclear reactions obey following conservation laws
(a) conservation of linear momentum
(d) conservation of number of nucleons.
(b) conservation of total energy
(e) conservation of angular momentum.
ILLUSTRATION-9.6
What is the power output of
Show Answer
SOLUTION:
Number of atoms in
fission rate
Each fission gives
Hence energy obtained in one second i.e. power output
= 58.46 mega-Watt
ILLUSTRATION-9.7
Calculate the binding energy per nucleon for
Show Answer
SOLUTION:
ILLUSTRATION-9.8
A star initially has
If average power radiated by star is
Show Answer
SOLUTION:
Adding the two equation
mass defect = 3
Power of star
Number of deutrons used per second
Time in which deutrons will be used
RADIOACTIVITY
The phenomenon of spontaneous emission of radiations from a substance is called radioactivity
Radioactivity was discovered by Henry Becquerel in 1896 in Uranium salts. The substances like Uranium, Radium. Thorium, Polonium efc. which show radioactivity are called radioactive substances. Nuclei with
Types of radioactive processes
(a) Alpha decay
e.g.
Alpha particle consists of 2 neitrons, 2 protons and carries positive charge in magnitude 2 electrons. It is doubly ionized helium nuclei.
energy of
(b) Beta decay
(a) Electron emission (
The interaction responsible for
(b) Positron emission
(c) Gamma decay
e.g.
(d) Electron capture
This process takes place when nucleus has too many protons relative to number of neutrons. i.e.
Properties of
| Property | |||
|---|---|---|---|
| 1. Nature | These are doubly ionized helium atom charge mass |
These are beam of fast moving electrons positions charge |
These are electromagnetic radiations of high frequency and travel in form of photons. charge rest mass effective mass |
| 2. Velocity | Speed ranges between |
speed ranges from |
speed equals velocity of light |
| 3. Ionising power |
These have maximum ionizing power |
There ionizing power is less than more than |
There ionizing power is least (1) |
| 4. Penetration power |
The penetration power is smallest. Can only penetrate through |
Penetration power is about 100 times that of can penetrate through 1 mm thick Al sheet |
Penetration power is very large. Can penetrale about |
| 5. Range | Range is very small (few cms in air) |
Range is more than (few meters in air) |
Range is very large (many hundreds of meter in air) |
| 6. Nature of spectrum |
Line spectrum | continuous spectrum | line spectrum |
| 7. Interaction with matter |
produces heat | produces heat | produces photoelectric effect Compton effect, pair production |
| 8. Effect of electric and magnetic field |
Suffers small deflection | suffers large deflection | pass undeflected |
| 9. Effect of photo graphic plate and |
Affects photographic plate and produces fluorescense |
Affects photographic plate and produces fluorescence |
Affects photographic plate and produces fluorescence. |
SODDY AND FAJAN’S DISPLACEMENT LAWS
1. For
2. For
3. For
4. For
RUTHERFORD - SODDY LAWS
The disintegration of a radioactive substance is random and spontaneous. Radioactive decay is purely a nuclear phenomenon and is independent of any physical and chemical conditions. The radioactive decay follows first order kinetics, i.e., the rate of decay is proportional to the number of undecayed atoms in a radioactive substance at any time
Let
The fraction of active atoms remaining at time
The number of atoms that have decayed in time
The fraction of atoms that have decayed in time
Fig. 9.12
Decay Constant
Decay Constant
Decay Constant is rate of decay of radioactive atoms per active atom.
At
The decay constant of radioactive element is equal to reciprocal of the time after which number of remaining active atoms reduce to
Unit of decay constant is second
If their are more than one radioactive elements in a group then the resultant decay Constant is equal to sum of individual decay constants.
Half life (T) :
Period of a radioactive substance is defined as the time in which one-half of the radioactive substance is disintegrated. If
From (i) and (ii)
Mean life
The mean life of an atom in a radioactive substance is called average life of radioactive substance.
Mean life
Thus mean life is equal to reciprocal of decay constant
Half life
Mean life is the time in which (a) number of active atoms reduces to
Mean life is equal to magnitude of reciprocal of slope of
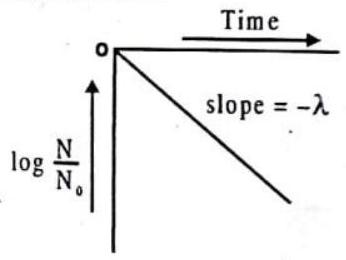
Fig. 9.13
ACTIVITY
The number of decays per unit time or decay rate is called activity.
Activity
where
The activity of one gram of radioactive substance called specific activity.
Units of activity
Curie: The specific activity of
1 curie
Rutherford :
1 rutherford
Becquerel :
I Becquerel
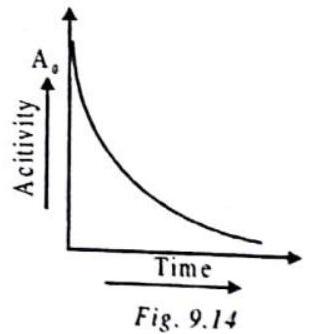
ILLUSTRATION-9.9
At any instant, the ratio of the amount of radioactive substances is
Show Answer
SOLUTION:
For first substance
For second substance,
Now,
ILLUSTRATION-9.10
Two radioactive substances
Show Answer
SOLUTION:
ILLUSTRATION-9.11
The half life of radium is 1620 years and its atomic weight is 226 . What will be the number of atoms that will decay from its
Show Answer
SOLUTION:
According to Avogadro’s hypothesis,
Half life
Because half life is very much large as compared to its time interval, hence
ILLUSTRATION-9.12
One gram of Radium emits
Show Answer
SOLUTION:
Rate of decay of Radium
or
Number of active atoms
or
Mean life
ILLUSTRATION-9.13
The activity of a radioactive substance drops to
Show Answer
SOLUTION:
Using
or
MISCELLANEOUS
SOLVED EXAMPLES
1. Find the ratio of the area of orbit of first excited state of electron to the area of orbit of ground level for hydrogen atom.
Show Answer
Solution.
2. The activity of a radioactive substance drops to
Show Answer
Solution. Using
i.e.
3. The half life of a radioactive substance is 34.65 minute. If
Show Answer
Solution. Activity
4. The mean life of a radioactive material for
Show Answer
Solution. There are two channels of decay so
or
The half life
5. A nucleus breaks into two parts whose velocity is in ratio 2:1. Find the ratio of their radius.
Show Answer
Solution. as per conservation of momentum
So
ratio of radii
so
6. The binding energy of
Show Answer
Solution. Given
7. The mass defect in a nuclear fusion reaction is
Show Answer
Solution. Total mass converted to energy
Energy liberated
8. Determine the power output of
Show Answer
Solution. Number of atoms in
Number of fission per second
Energy released per fission
9. If
Show Answer
Solution. Energy produced in one day
So input energy
energy released in one fission
no. of fissions per day
mass of
10. Ultraviolet light of wavelength
Show Answer
Solution. Let us find energy of each photon of given ultraviolet light
Maximum kinetic energy of emitted electron can be judged by stopping potential of 1.3 volt.
Using Einstein’s equation
Red light of wavelength
Thus energy of red light photons is less than work function
11. The total energy of an electron in the first excited state of the hydrogen atom is about
(a) What is the kinetic energy of the electron in this state?
(b) What is the potential energy of the electron in this state?
(c) Which of the answer above would change if the choice of the zero of potential energy is changed?
Show Answer
Solution. Kinetic energy of an electron in an orbit,
Potential energy of electron in the orbit
Total energy
It is given, total energy
(a) Kinetic energy of electron in this state
So,
(b) Potential energy
(c) If the zero of the potential energy is chosen differently, the kinetic energy remain the same. Althugh potential energy and hence total energy changes.
12. A radioactive isotope has a half-life of
Show Answer
Solution. Activity
Also instantaneous activity,
Initital activity,
So,
(a)
(b)
Required time, as can not be solved by direct calculation as in part (a).
13. The half-life of
Show Answer
Solution. Given
Mass
Number of atoms in
Activity of the sample,
14. Calculate the compare the energy released by (a) fusion of
Show Answer
Solution. (a) In the fusion reactions taking place within core of sun, 4 hydrogen nuclei combines to form a helium nucleus with the release of
Number of atoms in
Energy released inthe fusion of
(b) Energy released per fission of U-235 is
Number of atoms in
Total energy released for fission of
So the energy released infusion of
1 EXERCISE
Fill in the Blanks :
DIRECTIONS: Complete the following statements with an appropriate word / term to be filled in the blank space(s).
1. The energy released during a nuclear reaction is called ………….. energy
Show Answer
Answer: nuclear2. The nuclear fuel in the sun is …………..
Show Answer
Answer: hydrogen3. U-238 has ………….. neutrons.
Show Answer
Answer: 1464. In a nuclear reactor, nuclear energy is converted into …………..
Show Answer
Answer: electrical5. If the binding energy per nucleon for
Show Answer
Answer: 39.26. In plants and animals, the ratio of
Show Answer
Answer: a constant, i.e.,7. The radioactive radiation which can be stopped easily is …………..
Show Answer
Answer:8. When an a-particle is ejected, the atomic number of the atom decreases by …………..
Show Answer
Answer: 29. The cathode rays emitted from a concave cathode meet at …………..
Show Answer
Answer: centre of curvature10. Energy released in a nuclear reaction is given by …………..
Show Answer
Answer: E=11. ………….. are used to absorb the neutrons in a nuclear reactor.
Show Answer
Answer: cadmium rods12. The first experiment on radioactivily was done with the element …………..
Show Answer
Answer: radiumTrue / False
DIRECTIONS: Read the following statements and write your answer as true or false.
1. Atom bomb is based on the property of fission.
Show Answer
Answer: True2. High energy neutrons are used in a nuclear reactor to initiate the fission reaction?
Show Answer
Answer: False3. Cadmium rods are used as moderators in a nuclear reactor.
Show Answer
Answer: False4. Higher the mass defect, higher will be the stability of the nucleus.
Show Answer
Answer: False5. The e/m ratio of anode rays is constant irrespective of the gas filled inside the discharge tube.
Show Answer
Answer: False6. Thorium series is also called 4n series.
Show Answer
Answer: True7. Radioactive isotope of carbon is
Show Answer
Answer: True8. The radioactive isotope used in the treatment of cancer is a good source of
Show Answer
Answer: True9. In all radioactive series, the end element formed is lead.
Show Answer
Answer: True10. 4n + 2 series is called Uranium series.
Show Answer
Answer: TrueMatch the Following :
DIRECTIONS : Each question contains statements given in two columns which have to be matched. Statements
1. Column II give fission probability relative to
| Column I | Column II | ||
|---|---|---|---|
| (A) | (p) | 0.001 | |
| (B) | (q) | 1 | |
| (C) | (r) | 1.5 | |
| (D) | (s) | 0.0002 |
Show Answer
Answer: (A)2. Match the following
| Column I | Column II | ||
|---|---|---|---|
| (A) | Coolidge tube | (p) | present in all natural radioactive series |
| (B) | (q) | istones | |
| (C) | Radon | (r) | decrease in atomic number by two |
| (D) | (s) | X-rays |
Show Answer
Answer: (A)3. Match the following.
| Column I | Column II | ||
|---|---|---|---|
| (A) | Binding energy per nucleon | (p) | |
| (B) | Moderator | (q) | to slow down |
| (C) | Thermonuclear reaction | (r) | nuclear fusion |
| (D) | Mass energy equivalence | (s) | Stability of nucleus |
Show Answer
Answer: (A)Very Short Answer Questions :
DIRECTIONS : Give answer in one word or one sentence.
1. In a discharge tube, at what pressure cathode rays are produced?
Show Answer
Answer:2. If the half-life period of a radioactive substance is 0.696 years, what is its decay constant?
Show Answer
Answer:3. What happens when cathode rays are suddenly stopped?
Show Answer
Answer: X-rays are produced.4. How can we say that cathode rays consist of negatively charged particles?
Show Answer
Answer: Direction of deflection in electric and magnetic fields.5. Define binding energy.
Show Answer
Answer: Energy that results from the loss of mass when nucleus is formed.6. How are
Show Answer
Answer: When cathode rays are suddenly stopped by a metal target.7. What forces are responsible for keeping the nucleons together in a nucleus?
Show Answer
Answer: Nuclear forces are responsible.8. Which rays among
Show Answer
Answer: (i)9. What is a moderator? Give one example.
Show Answer
Answer: Used to slow down neutrons. Heavy water.10. What is radioactive transformation or radioactive decay?
Show Answer
Answer: Disintegration of an unstable nucleus resulting in the formation of another nucleus.11. Name the three radioactive series. Which series is referred to as
Show Answer
Answer: (i) Uranium series, actino-uranium series and thorium series.
12. What is a solar cell?
13. What are the advantages and disadvantages of a solar cell?
14. What energy transformation takes place in a solar cell?
15. What factors make a solar cell very expensive?
16. What is a solar panel?
17. Name the scientist who gave correct explanation of the source of sun’s energy.
Show Answer
Answer: Hans Bethe18. What is the surface temperature of the sun?
Show Answer
Answer:19. Name the fuel used in atomic reactors.
Show Answer
Answer:20. How much energy is released by one atomic mass unit of substance?
21. Name the process that forms the principle of a nuclear
Show Answer
Answer: (i) Nuclear fission22. Which of the two processes is carried out at a higher temperature: nuclear fission or nuclear fusion?
Show Answer
Answer:
Nuclear fusion
23. What do you understand by nuclear wastes?
Show Answer
Answer: The discarded or unwanted materials of nuclear industry are all called nuclear wastes.Short Answer Questions :
DIRECTIONS : Give answer in 2-3 sentences.
1. Define nuclear fission and fusion reactions.
2. What are advantages and disadvantages of nuclear reactors?
3. Mention any three harmful effects of nuclear radiation on our body.
Show Answer
Answer: (i) Damages of tissues to chromosomes enhancing mutation.
(ii) Initiation of unwanted cell division leading to cancerous growth.
(iii) Conversion of molecules of living cells into chemicallyreactive ions, which disrupt cell membranes resulting in serious illness.
4. A tremendous amount of energy is released during a nuclear fission reaction. Why?
Show Answer
Answer: In a nuclear reaction, the mass of the reactants is more than the mass of the products. Thus, the loss of mass in a nuclear reaction is converted into energy according to the massenergy equivalence equation,5. Give two applications of nuclear fission.
Show Answer
Answer: (i) The energy emitted during nuclear fission can trapped for peaceful uses like the generation of electricity.
(ii) It is used in warfare-for making atomic bombs which are produced by uncontrolled nuclear fission.
6. What would happen if all the hydrogen present in the sun is converted into helium?
Show Answer
Answer: In case all the hydrogen present in the sun is converted into helium, the temperature and pressure inside the sun will decrease. As a result of this, the sun will collapse due to its own gravity. When the size of sun decreases, its interior temperature will again rise and hydrogen nuclei will get liberated from the helium nuclei.7. It is said that the energy produced from nuclear fusion would create fewer pollution problems than the energy produced by nuclear fission. Explain why?
Show Answer
Answer: The products obtained in nuclear fusion are not radioactive. Since they are not harmful, they can be disposed off easily. On the other hand, the products and by-products given out in nuclear fission are radioactive and hence are dangerous as they pollute water and air.8. Cadmium rods play an important role in a nuclear reactor. What happens
(i) when they are completely inserted into nuclear fuel and
(ii) when they are slowly withdrawn from the nuclear fuel?
Show Answer
Answer: (i) All neutrons are absorbed, and the chain reaction stops. (ii) Correct number of neutrons are left behind to sustain the chain reaction and the reactor becomes critical.9. “Obtaining of energy from nuclear fusion reactions is preferable to obtaining of energy from nuclear fission reactions”. Give two reasons to justify this statement.
Show Answer
Answer: (i) Nuclear fusion generates more energy than nuclear fission. (ii) The products formed in fusion reactions are nonradioactive and do not create radioactive pollutions.10. What are radioactive isotopes? Mention two harmful effects of nuclear radiations.
Show Answer
Answer: Radioactive isotopes are those isotopes which release. spontaneously,11. What is nuclear fusion reaction? Why are such reaction not possible in the school laboratory? State the amount of energy released by one gram of hydrogen in the sun.
Show Answer
Answer: Nuclear fusion reaction is the reaction in which lighter nuclei combine to form heavier nucleus, with the release of large amount of energy. These reactions are not possible in school laboratory, since the energy released is quite high and difficult to handle.
One gram of hydrogen releases
12. Name the process involved in the liberation of energy in (i) the sun and (ii) a nuclear reactor. Mention any two differences between the two processes.
Show Answer
Answer: (i) Fusion of Hydrogen, (ii) Fission.
Fission has been controlled and forms radioactive products and lighter elements. Fusion produces energy which is uncontrollable and heavier elements.
13. Indicate in symbols for any three isotopes of uranium. Which one among these is used for nuclear fission? What is meant by criticality of a nuclear reaction?
Show Answer
Answer: Isotopes of Uranium
When sufficient
14. What is the cause of release of unusually large energies in nuclear fission reactions? How is the energy per fission calculated?
Show Answer
Answer: The mass of nuclei obtained after the fission is less than the mass of the disintegrated nucleus. This difference in mass i.e., lost mass reappears in the form of large energy at a rate governed by the Einstein mass-energy relation.
To find energy per fission.
(i) Find the mass of reactants in kg or amu.
(ii) Find the mass of the nuclei obtained after fission in anu.
(iii) Find the difference in mass called mass defect
Long Answer Questions :
DIRECTIONS : Give answer in four to five sentences.
1. Name the main components of the nuclear reactor and describe their function.
Show Answer
Answer: (i) A core [For nuclear fuel]
(ii) A moderator [For slowing down fast neutrons]
(iii) Control rods [To absorb (or remove) extra neutrons]
(iv) Coolant or heat exchanger [For transferring heat from the core]
(v) A Protective shield [To prevent harmful radiations from escaping to the surroundings]
Core : It contains nuclear fuel, which undergoes nuclear fission very rapidly to generate energy. Fuels commonly used in nuclear reactors are uranium-235 (U-235) and plutonium-239 (Pu-239).
Nuclear fuel : The nuclear fuel used in nuclear reactors is usually enriched
Moderator : The neutrons released are fast neutrons having energy of about
Control rods : The rate of reaction is controlled by inserting or withdrawing rods made of elements whose nuclei absorb neutrons without undergoing fission. These rods are called control rods. Typical examples of control rods are those made of boron or cadmium.
Coolant : The purpose of the coolant is to carry the energy of the fission reaction from the core of the reactor and take it to a place of utilisation; i.e., a steam generator. The commonly used coolants are heavy water, molten sodium, molten potassium and pressurised
Protective shield : To stop the spreading of the harmful radiations from a nuclear reactor, the reactor is enclosed in thick concrete walls, often
2. Compare the energy during fission and fusion. What is the advantage of fusion over fission?
Show Answer
Answer: The energy given out during fusion is about seven times the energy given out during fission for the same mass. The advantage of the fusion reaction over the fission reaction is that the energy produced by fusion is clean and is not accompanied by the generation of any hazardous radioactive waste. Nuclear Fusion also has the following advantages over nuclear fission
(i) For the same masses of the reacting substances involved, fusion releases much more energy than fission.
(ii) The products of fusion reaction are not radioactive and are, therefore, easier to dispose off as compared to the products formed by fission reaction.
(iii) Nuclear fission requires urarium as a fuel which is limited and exhaustible substance, while nuclear fusion requires deuterium which can be obtained abundantly from sea water.
3. A radioactive sample is kept at the centre of a large evacuated sphere. How safe will it be?
Show Answer
Answer: For safety, the radiations4. .Explain Bohr’s atomic model.
5. Explain in detail the nuclear hazards and the safety precautions to be taken.
6. Describe how cathode rays are produced in a discharge tube. Write down the properties and uses of cathode rays.
7. Describe in detail the process of
8. Compare the properties of
2 EXERCISE
Multiple Choice Questions :
DIRECTIONS : This section contains multiple choice questions. Each question has 4 choices (a), (b), (c) and (d) out of which ONLY ONE is correct.
1. In a nuclear power plant, uranium atoms
(a) combine and give off heat energy
(b) split and give off heat energy
(c) burn and give off heat energy
(d) split and give off electrons
Show Answer
Answer: (b)2. Solar energy is produced by the following reaction
(a) Fission reaction
(b) Fusion reaction
(c) Chemical reaction
(d) None of the above
Show Answer
Answer: (b)3. The volume occupied by an atom is greater then the volume of the nucleus by a factor of about
(a)
(c)
(b)
(d)
Show Answer
Answer: (c)4. Which of the following is true for isotopes of specimen of
(a) both contain same number of neutrons
(b) both contain same of number of proton, electron and neutron
(c) both contain same number of proton and electron but
(d)
Show Answer
Answer: (c)5. Atomic nucleus contains
(a) electron & photon
(b) electron, proton & neutron
(c) electron & neutron
(d) proton & neutron
Show Answer
Answer: (d)6. The atomic number & mass number of element is
(a)
(c)
(b)
(d)
Show Answer
Answer: (d)7. Nuclei containing different number of protons but same number of neutrons are called
(a) Iso clinics
(c) isotones
(b) isobars
(d) isotopes
Show Answer
Answer: (c)8. 1 amu is equivalent to
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)9. The dependence of density [d] of nuclear matter on the mass number
(a)
(b)
(c)
(d)
Show Answer
Answer: (c)10. The wrong statement is
(a) Nuclear forces are strongest
(b) Nuclear forces are very short range forces
(c) Nuclear force increase when the number of nucleons is increased
(d) Nuclear force is produced by the exchange of pions
Show Answer
Answer: (c)11. Range of nuclear force is approximately
(a)
(b)
(c)
(d)
Show Answer
Answer: (d)12. The mass number of a nucleus is equal to the number of
(a) Electron it contains
(b) Protons it contains
(c) Neutrons it contains
(d) Nucleons it contains
Show Answer
Answer: (d)13. The neutron was discovered by
(a) Marie Curie
(b) Pierre Curie
(c) James Chadwick
(d) Rutherford
Show Answer
Answer: (c)14. The order of magnitude of the density of nuclear matter is
(a)
(c)
(b)
(d)
Show Answer
Answer: (b)15. Force between protons in nucleus will be
(a) only nuclear
(b) only coulomb
(c) nuclear & coulomb
(d) coulomb & gravitational
Show Answer
Answer: (c)16. The mass equivalent of
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)17. Boron rods are used in nuclear reactor as
(a) moderator
(b) control rods
(c) coolant
(d) protective shield
Show Answer
Answer: (b)18. Best moderator for neutron is
(a) berillium oxide
(b) pure water
(c) heavy water
(d) graphite
Show Answer
Answer: (c)19. Nuclear fission was discovered by
(a) OttoHahn and strassman
(b) Fermi
(c) Bethe
(d) Rutherford
Show Answer
Answer: (a)20.
(a) 1000
(c)
(b)
(d) 931
Show Answer
Answer: (c)21. In the process of nuclear fusion
(a) Only heavy nucleus break into light nuclei
(b) Fusion of light nuclei at normal temperature
(c) Fusion of light nuclei at high pressure and low temperature
(d) Fusion of light nuclei at high pressure and high temperature
Show Answer
Answer: (d)22. When
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)23. The cause of energy liberated in nuclear reaction is
(a) Change of potential energy into kinetic energy
(b) Kinetic energy of resultant nucleus
(c) Energy equivalent to mass lost
(d) None of these
Show Answer
Answer: (c)24. Atom bomb consists of pieces of
(a) Proton
(b) Neutron
(c) Meson
(d) Electron
Show Answer
Answer: (b)25. When four hydrogen nuclei fuse together to form helium nucleus, then in this process
(a) Energy is absorbed.
(b) Energy is liberated.
(c) Absorption and liberation of energy depends upon the temperature.
(d) Energy is neither liberated nor absorbed.
Show Answer
Answer: (b)26. Two lighter nuclei are fused together to form a nucleus of medium atomic mass and energy is released in this process because
(a) Binding energy of lighter nuclei is more.
(b) Binding energy per nucleon of lighter nuclei is more.
(c) Binding energy per nucleon of medium nucleus is more.
(d) Energy is always released when two nuclei are fused.
Show Answer
Answer: (c)27. Neutron ratio (available/used per fission in atomic reactor and atom bomb are
(a)
(b)
(c)
(d)
Show Answer
Answer: (b)28. In atomic explosion, a temperature of about 10 million degrees is developed at the moment of explosion. The wavelength of light coming from the hot region of the atomic explosion lie in the region
(a) ultraviolet region
(b) visible region
(c) infrared region
(d)
Show Answer
Answer: (d)29. When light is incident on surface, photo electrons are emitted. For photoelectrons
(a) The value of kinetic energy is same
(b) Kinetic energy does not depend on the wave length of incident light
(c) The value of kinetic energy is equal to or less than a maximum energy
(d) None of the above
Show Answer
Answer: (c)30. The phenomenon of photo electric emission depends on
(a) Only wave length of incident light
(b) Only work function of surface
(c) Only nature of surface
(d) All of the above
Show Answer
Answer: (d)31. Photo electric effect is the phenomenon in which
(a) Photons come out of a metal when it is hit by a beam of electrons
(b) Photons come out of the nucleus of an atom under the action of an electric filed
(c) Electrons come out of metal with a constant velocity depending on frequency and intensity of incident light
(d) Electrons come out of a metal with different velocity not greater than a certain value which depends only on the frequency of the incident light wave and not on its intensity.
Show Answer
Answer: (c)32. The electrons are emitted in the photo electric effect from a metal surface
(a) Only if the frequency of radiation is above a certain threshold value
(b) Only if the temperature of the surface is high
(c) At a rate that is independent of the nature of metal
(d) With a maximum velocity which is proportional to the frequency of incident radiation
Show Answer
Answer: (a)33. Work function is
(a) Energy necessary to eject the electron from its orbit
(b) Energy necessary to eject the electron from metal
(c) Minimum necessary energy to eject the electron from metal
(d) Wavelength necessary for releasing an electron from a body
Show Answer
Answer: (c)34. Einstein got noble prize for
(a) Photo electric effect
(b) Compton effect
(c) Theory of relativity
(d) None of the above
Show Answer
Answer: (a)35. In a nuclear reactor, the moderator is
(a) uranium-234
(b) uranium-238
(c) cadmium
(d) heavy water
Show Answer
Answer: (d)36. Which of the following is a good nuclear fuel?
(a) uranium-236
(b) neptunium-239
(c) thorium-236
(d) plutonium-239
Show Answer
Answer: (d)37. A radioactive substance has a half life of four months. Three fourth of the substance will decay in
(a) Three months
(b) Four months
(c) Eight months
(d) Twelve months
Show Answer
Answer: (c) Substance left undecayed -
38. When hydrogen atom is in its first excited level, it’s radius is
(a) Four times, it ground state radius
(b) Twice times, it ground state radius
(c) Same times, it ground state radius
(d) Half times, it ground state radius.
Show Answer
Answer: (a)
39. For a nuclear fusion process, suitable nucli are
(a) Any Nuclei
(b) Heavy Nuclei
(c) Light Nuclei
(d) Nuclei lying in the middle of periodic table
Show Answer
Answer: (c)40. If
(a)
(b)
(c)
(d)
Show Answer
Answer: (a) Amount left41. If mass-energy equivalence is taken into account, when water is cooled to form ice, the mass of water should
(a) increase
(b) remain unchanged
(c) decrease
(d) first increase then decrease
Show Answer
Answer: (a) Because thermal energy decreases, therefore mass42. Which of the following cannot be emitted by radioactive substances during their decay?
(a) Protons
(b) Neutrinoes
(c) Helium nuclei
(d) Electrons
Show Answer
Answer: (a)More Than One Correct :
DIRECTIONS : This section contains multiple choice questions. Each question has 4 choices (a), (b), (c) and (d) out of which ONE OR MORE may be correct.
1.
(a) 0.51 MeV
(b)
(c) 0.0005477 amu
(d) none of these
Show Answer
Answer: (b,c)2. According to Bohr’s theory of hydrogen atom, for the electron in the
(a) linear momentum is proportional to
(b) radius is proportional to
(c) the kinetic energy is proportional to
(d) the angular momentum is proportional to
Show Answer
Answer: (a,c,d)3. An electron orbiting in a circular orbit around the nucleus of an atom
(a) has a magnetic dipole moment
(b) exerts an electric force on the nucleus equal to that on it by the nucleus
(c) does produces a magnetic induction at the nucleus
(d) has a net energy inversely proportional to its distance from the nucleus
Show Answer
Answer: (a,b,c,d)4. A particular hydrogen like atom has its ground state binding energy
(a) its atomic number is 3
(b) an electron witit
(c) an
(d) an electron of
Show Answer
Answer: (a,c,d)5. In which of the following situations the heavier of the two particles has smaller de Broglie wavelength? The two particles
(a) move with the same speed
(b) move with the same linear momentum
(c) move with the same kinetic energy
(d) have fallen through the same height
Show Answer
Answer: (a,c,d)6. In the hydrogen atom in the ground state
(a) the kinetic energy of the electron is less than the potential energy which is positive
(b) the potential energy is less than the kinetic energy which is positive
(c) the potential energy is negative and the kinetic energy numerically less than the numerical value of potential energy
(d) the total energy is negative
Show Answer
Answer: (c,d)7. Mark the correct options
(a) An atom with a vacancy has a smaller than the neutral atom
(b)
(c) The wavelength of
(d) The wavelength of
Show Answer
Answer: (b,c)8. Who was/were won the novel prize of physics in 1903?
(a) Pierre Curie
(b) Becquerel
(c) Rutherford
(d) Madame Curie
Show Answer
Answer: (a,b)9. When the nucleus of an electrically neutral atom undergoes a radioactive decay process, it will remain neutral after the decay if the process is
(a) an
(b) a
(c) a
(d) a
Show Answer
Answer: (c,d)10. When a nucleus with atomic number
(a) both
(b)
(c)
(d)
Show Answer
Answer: (a,b,c,d)11. During a
(a) The daughter nucleus has one proton less than the parent nucleus
(b) The daughter nucleus has one proton more than the parent nucleus
(c) An electron which is already present within the nucleus is rejected
(d) A neutron in the nucleus decays emitting an electron
Show Answer
Answer: (b,d)12. The decay constant of a radioactive substance is 173
(a) nearly
(b) half-life of the radioactive substance is (1/0.173) year
(c) one-fourth of the radioactive substance will be left after 8 years
(d) all the above statements are true
Show Answer
Answer: (a,c)13. Which of the following reactions are not possible?
(a)
(b)
(c)
(d)
Show Answer
Answer: (a,d)14. The probability of disintegration per second of a nucleus in a given radioactive sample
(a) increases proportional to the life time lived by the nucleus
(b) decreases with the life time lived
(c) is independent of the life time lived
(d) depends on the total number of identical nuclei present in the sample
Show Answer
Answer: (c,d)15. The heavier nuclei tend to have larger
(a) a neutron is heavier than a proton
(b) a neutron is an unstable particle
(c) a neutron does not exert electric repulsion
(d) Coulomb force has longer range as compared to the nuclear force
Show Answer
Answer: (c,d)16.
(a) the probability of a nucleus disintegrating during one second is lower in the first half-life and greater in the fifth half-life
(b) the probability of a nucleus disintegrating during one second remains constant for all the time
(c) quite an appreciable quantity of
(d) the energy of emitted
Show Answer
Answer: (b,c,d)17. During the radioactive decay
(a) atomic mass number cannot increase
(b) atomic number may increase
(c) atomic number may decrease
(d) atomic number may remain unchanged
Show Answer
Answer: (a,b,c,d)18. An electron in a hydrogen atom makes a transition
(a)
(c)
(b)
(d)
Show Answer
Answer: (a,d)Fill in the Passage :
DIRECTIONS : Fill in the blanks in the following passage(s) from the words given inside the box.
I.
release
fissions
Atomic Bomb, powerful explosive nuclear weapon fueled by the splitting, or fission, of the nuclei of specific ……..1…….. of uranium or plutonium in a ……..2…….. The strength of the ……..3…….. created by an atomic bomb is on the order of the strength of the explosion that would be created by thousands of tons of TNT (Trinitrotoluene).
An atomic bomb must provide enough mass of plutonium or uranium to reach ……..4…….., the mass at which the nuclear reactions going on inside the material can make up for the neutrons leaving the material through its outside surface. Usually the plutonium or uranium in a bomb is separated into parts so that critical mass is not reached until the bomb is set to explode. At that point, a set of chemical explosives or some other mechanism drives all the different pieces of uranium or plutonium together to ……..5…….. a critical mass. After this occurs, there are enough neutrons bouncing around in the material to create a chain reaction of ……..6…….. . In the fission reactions, collisions between neutrons and uranium or plutonium atoms cause the atoms to split into pairs of nuclear fragments, releasing energy and more neutrons. Once the reactions begin, the neutrons ……..7…….. by each reaction hit other atoms and create more fission reactions until all the fissile material is exhausted or scattered.
Show Answer
Answer: 1. isotopes; 2. chain reaction; 3. explosion; 4. critical mass: 5. produce; 6 . fissions; 7. releaseII.
atom bomb
temperature
harmful
Another form of nuclear energy is called fusion. Fusion means joining smaller nuclei to make a larger nucleus. The sun uses nuclear fusion of ……..1…….. atoms into ……..2…….. atoms. This gives off heat and light and other radiation. Also given off in this fusion reaction is energy.
Scientists have been working on controlling nuclear fusion for a long time, trying to make a fusion reactor to produce electricity. But they have been having trouble learning how to control the reaction in a contained space.
Controlled nuclear fusion is believed by many scientists to be the ultimate solution to the world’s energy problems. The energy released in ……..3…….. reactions is many times greater than that released in ……..4…….. reactions. To date, however, the technology has not been developed to make use of this source of energy. What’s better about nuclear fusion is that it creates less radioactive material than fission, and its supply of fuel can last longer than the sun
Fusion is possible at high pressure (
The proton-proton cycle happens at lower temperature as compared to carbon-nitrogen cycle.
Nuclear fusion in possible at a place which has reactants in large quantity.
……..5…….. works on principle of nuclear fusion.
The explosion of a hydrogen bomb needs an explosion of ……..6…….. to generate required ……..7……..
No ……..8…….. radiations are produced in fusion.
Show Answer
Answer: 1. hydrogen; 2. helium; 3. fusion; 4. Fission; 5. Hydrogen bomb; 6. atom bomb; 7. temperature; 8. harmfulPassage Based Questions :
DIRECTIONS : Study the given paragraph(s) and answer the following questions.
PASSAGE-I
A physicist wishes to eject electrons by shining light on a metal surface. The light source emits light of wavelength of
1. Which metal(s) can be used to produce electrons by the photoelectric effect from given source of light?
(a) Barium only
(b) Barium or lithium
(c) Lithium, tantalum or tungsten
(d) Tungsten or tantalum
Show Answer
Answer: (b)
For photoelectric effect,
2. Which option correctly identifies the metal that will produce the most energetic electrons and their energies?
(a) Lithium,
(b) Tungston,
(c) Lithium,
(d) Tungston,
Show Answer
Answer: (a)
For maximum value of
3. Suppose photoelectric experiment is done separately with these metals with light of wavelength
(a) 2.75 volt
(b) 4.5 volt
(c) 0.45 volt
(d) 0.25 volt
Show Answer
Answer: (c) The.maximum magnitude of stopping potential will be for metal of least work function.
PASSAGE-II
Gold nucleus
Atomic masses :
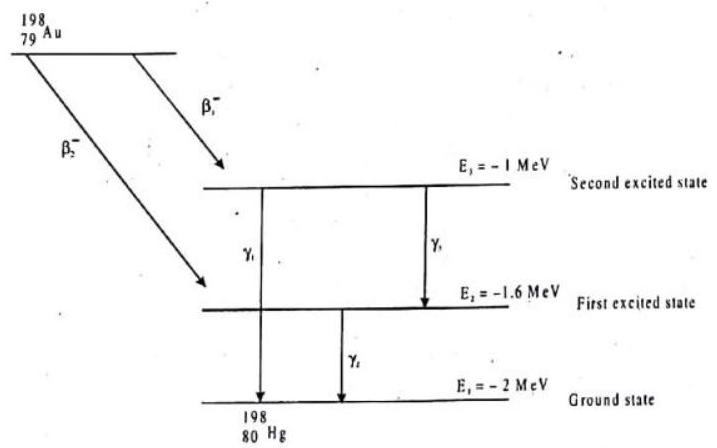
1. What is the maximum kinetic energy of emitted
(a)
(b)
(c)
(d)
Show Answer
Answer: (d) Total energy released from
Energy released from
2. What is the maximum kinetic energy of emitted
(a)
(b)
(c)
(d)
Show Answer
Answer: (d) Similarly maximum kinetic energy of3. The wavelength of emitted
(a)
(b)
(c)
(d)
Show Answer
Answer: (a)Assertion & Reason :
DIRECTIONS : Each of these questions contains an Assertion followed by Reason. Read them carefully and answer the question on the basis offollowing options. You have to select the one that best describes the two statements.
(a) If both Assertion and Reason are correct and Reason is the correct explanation of Assertion.
(b) If both Assertion and Reason are correct, but Reason is not the correct explanation of Assertion.
(c) If Assertion is correct but Reason is incorrect.
(d) If Assertion is incorrect but Reason is correct.
1. Assertion : Nuclear forces are independent of charges.
Reason : Nuclear force is not a central force.
Show Answer
Answer: (b)2. Assertion : The strength of photoelectric current depends upon the intensity of incident radiation.
Reason : A photon of energy
Show Answer
Answer: (b)3. Assertion : Binding energy (or mass defect) of hydrogen nucleus is zero.
Reason : Hydrogen nucleus contain only one nucleon.
Show Answer
Answer: (a)4. Assertion :
Reason : During nuclear fission a part of the original mass of
Show Answer
Answer: (a)5. Assertion : The rest mass energy of a nucleus is smaller than the rest mass energy of its constituent nucleons in free state.
Reason : Nucleons are bound together in a nucleus.
Show Answer
Answer: (a)6. Assertion : In a decay process of a nucleus, the mass of products is less than that of the parent.
Reason : The rest mass energy of the products must be less than that of the parent.
Show Answer
Answer: (a)7. Assertion : In street light circuits, photo-cells are used to switch on and off the lights automatically at dusk and dawn.
Reason : A photocell can convert a change in intensity of illumination into a change in photocurrent that can be used to control lighting system.
Show Answer
Answer: (a)Multiple Matching Questions :
DIRECTIONS : Following question has four statements
1. Match the processes in column I with their properties in Column II.
| Column I | Column II | ||
|---|---|---|---|
| (A) | Nuclear fission | (p) | involves weak nuclear forces |
| (B) | Nuclear fusion | (q) | involves conversion of matter into energy |
| (C) | (r) | atoms of higher atomic number are used | |
| (D) | Exothermic nuclear reaction | (s) | atoms of lower atomic reaction number are used |
Show Answer
Answer:2. Match the following columns
| Column I | Column II | ||
|---|---|---|---|
| (A) | Nuclear fission | (p) | Converts some matter into energy |
| (B) | Nuclear fusion | (q) | Generally possible for nuclei with low atomic no. |
| (C) | (r) | Generally possible for nuclei with higher atomic number | |
| (D) | Exothermic nuclear | (s) | Essentially proceeds by weak nuclear forces |
Show Answer
Answer:3. Match the entries of column I with the entries of column II.
| Column I | Column II | ||
|---|---|---|---|
| (A) | The sun | (p) | Nuclear fission |
| (B) | Nuclear reactor reaction | (q) | Nuclear fusion |
| (C) | Total binding energy in a process is increased | (r) | Energy is released |
| (D) | Total binding energy in a process is decreased | (s) | Energy is absorbed |
Show Answer
Answer:HOTS Subjective Questions :
DIRECTIONS : Answer the following questions.
1. Name any one element that is used in making solar cells. On what property of the element in this use based?
Show Answer
Answer: Germanium or Silicon. The use is based on the semiconducting property.2. Write any two harmful radiations emitted by nuclear wastes.
Show Answer
Answer:3. Mention any two harmful effects of nuclear radiations on human body.
Show Answer
Answer: (i) Genetical disorders (ii) Skin cancer.4. The mass number of elements
Show Answer
Answer: (a) Atom bomb
(b) Hydrogen bomb
5.
Show Answer
Answer: Energy
Energy per fission
Number of fissions
6. If
Show Answer
Answer: Energy released per hour
Energy released in 10 minutes
7. What is a nuclear reactor? What is a moderator? State the functions of moderator in a nuclear reactor. Name two substances used as moderators.
Show Answer
Answer: Nuclear reactor is a device where a nuclear fission reaction is carried out to generate electricity. Moderators are substances which slow down the neutrons and make them fit enough to induce fission in8. State the laws of radioactive emissions.
Show Answer
Answer:
Where
9. Find the binding energy per nucleon for a lithium nucleus
Show Answer
Answer: (i) Mass defect
(ii) Binding energy
(iii) Binding energy per nucleon
10. What are prompt fission and delayed fission reactions?
Show Answer
Answer: (i) Fission takes place as soon as the heavy nucleus is bombarded with a neutron.
(ii) Neutron enters a heavy nucleus causing instability and eventually leading to the fission.
11. Mention the properties of cathode rays.
Show Answer
Answer: (i) Travel in straight line.
(ii) Exert mechanical force on the objects.
(iii) Deflect in presence of electric and magnetic fields.
(iv) Ionize the gas through which they pass.
(v) Affect photographic plates.
12. What is nuclear fusion? What are the conditions for a nuclear fusion reaction to take place?
Show Answer
Answer: (i) Two or more lighter nuclei combine to give a heavy nucleus.
(ii) High temperature.
13. Explain nuclear chain reactions.
Show Answer
Answer: (i) A series of nuclear fissions where by the neutrons produced in each fission cause additional fission, releasing enormous amount of energy.14. Explain why the energy released during fusion cannot be used to produce electricity.
Show Answer
Answer: (i) fusion reactions are not chain reactions - Not possible to sustain the reaction
(ii) Requires a high temperature for nuclear reaction to take place.
(iii) Difficult to harness and convert the heat energy produced into mechanical energy.
15. Mention the properties of
Show Answer
Answer: (i) Electromagnetic radiations.
(ii) Wavelength is of the order of
(iii) Affect photographic plates
(iv) Travel with velocity of light in straight line
(v) Produce fluorescence in certain materials.
(vi) Not affected by electric and magnetic fields.
16. What is a nuclear reactor? Name its different parts.
Show Answer
Answer: (i) A device, in which a self-sustaining controlled chain reaction is produced in a fissionable material
(ii) Nuclear fuel, moderator, coolant, shield, control rods.
17. Give any three uses of radio-isotopes.
Show Answer
Answer: (i)
(ii)
(iii)
18. Explain nuclear fission by giving an example.
Show Answer
Answer: (i) A process in which a heavy nucleus of a radioactive substance like uranium is split into lighter nuclei by the bombardment of a low energy neutrons.
(ii)
19. Using
Show Answer
Answer:20. What is a radioactive series? How is it formed? Explain.
Show Answer
Answer: (i) Sequential decay of a radioactive nucleus.
(ii) Results in the production of new nuclei at each stage.
(iii) Gives rise to a series of elements called radioactive decay series.










