JEE Main 12 Jan 2019 Morning Question 17
Question: For a diatomic ideal gas in a closed system, which of the following plots does not correctly describe the relation between various thermodynamic quantities? [JEE Main Online Paper Held On 12-Jan-2019 Morning]
Options:
A)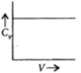
B)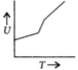
C)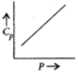
D)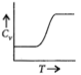
Show Answer
Answer:
Correct Answer: C
Solution:

We know value of ${C_v}$ is independent of volume (V) and depends in T i.e.at a constant temperature for any given value of V we have same value of ${C_v}$.
So the graph between C_v and V will be parallel to X-axis.Similarly the value of ${C_p}$ is independent of P and depends on T
i.e. at constant temperature for any given value of P we have same value of ${C_p}$ .So, we can say graph b is incorrect.
Also at higher temperatures, the rotational degree of freedom becomes active. So, with the increase in temperature the internal energy and heat capacity increases.










