JEE Main On 16 April 2018 Question 28
Question: One mole of an ideal monoatomic gas is taken along the path ABCA as shown in the PV diagram. The maximum temperature attained by the gas along the path BC is given by [JEE Main 16-4-2018]
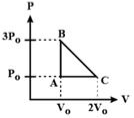
Options:
A) $ \frac{25}{8}\frac{P _0V _0}{R} $
B) $ \frac{25}{4}\frac{P _0V _0}{R} $
C) $ \frac{25}{16}\frac{P _0V _0}{R} $
D) $ \frac{5}{8}\frac{P _0V _0}{R} $
Show Answer
Answer:
Correct Answer: A
Solution:
Temperature at A = $ \frac{3P _0V}{nR} $
Temperature at $ b=\frac{P _0\times 2V _0}{nR} $
Maximum temperature can be between B and C
P-V equation for process BC $ P-3P _0=\frac{P _0-3P _0}{2V _0-V _0}\times (V-V _0) $
$ P-3P _0=\frac{-2P _0V}{V _0}+2P _0 $ $ P=\frac{-2P _0V}{V _0}+2P _0 $
Multiply by V $ PV=\frac{-2P _0V}{V _0}+5P _0 $ $ RT=-\frac{2P _0}{V _0}V^{2}+5P _0V $
Make $ \frac{dT}{dV}=0 $ This gives $ T=\frac{25P _0V _0}{8R} $










