JEE Main On 16 April 2018 Question 24
Question: The most polar compound among the following is: [JEE Main 16-4-2018]
Options:
A) 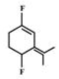
B) 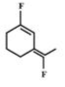
C) 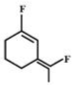
D) 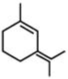
Show Answer
Answer:
Correct Answer: C
Solution:
- Among the substituent attached to the given compounds, Fluorine has maximum polarity. So it will push dipole moment towards itself. In compound in option A the two F groups are attached opposite to each other thus net dipole moment will cancel each other and reduce its polarity. In compound in option B the F groups are attached in slightly opposite direction thus this also decreases its polarity. But the compound in option C has the two F groups along same direction. Thus net dipole moment will increase in this direction thus it will have maximum polarity. Thus the compound in option C has maximum polarity.










