JEE Main On 08 April 2018 Question 11
Question: Which of the following lines correctly show the temperature dependence of equilibrium constant, K, for an exothermic reaction? [JEE Main Online 08-04-2018]
Options:
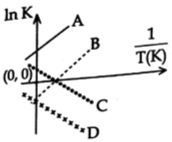
A) C and D
B) A and D
C) A and B
D) B and C
Show Answer
Answer:
Correct Answer: C
Solution:
- $ \text{InK=InA-}\frac{\Delta H}{RT}\text{,} $ For exothermic Reaction $ \Delta H=-ve $ slope $ =( -\frac{\Delta H}{R} )=+ve $ .










