JEE Main Solved Paper 2017 Question 16
Question: Which of the following compounds will behave as a reducing sugar in an aqueous KOH solution? [JEE Main Solved Paper-2017]
Options:
A) 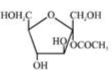
B) 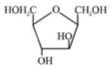
C) 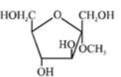
D) 
Show Answer
Answer:
Correct Answer: A
Solution:
- [a] Ester in presence of Aqueous KOH solution give SNAE reaction so following reaction takes place [b] In above compound in presence of Aq. KOH $ \text{(S}{N^{AE}}\text{)} $ reaction takes place $ \propto - $ Hydroxy carbonyl compound is formed which give $ \oplus ve $ Tollen’s test So this compound behave as reducing sugar in an aqueous KOH solution.










