Properties of Matter 4 Question 13
15. Two soap bubbles
(2009)
Show Answer
Answer:
Correct Answer: 15. 6
Solution:
- Although not given in the question, but we will have to assume that temperatures of
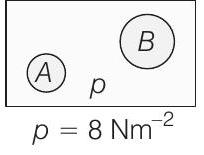
Substituting the values, we get






