Heat and Thermodynamics 5 Question 7
7. For the given cyclic process
(2019 Main, 12 Jan I)
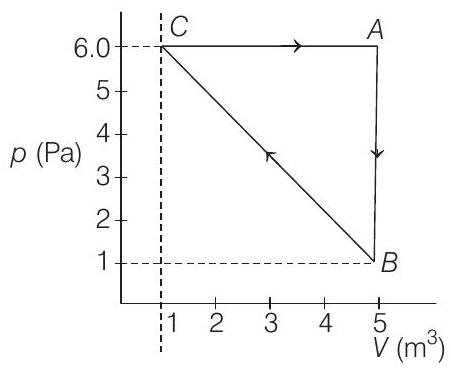
(a)
(b)
(c)
(d)
Show Answer
Answer:
Correct Answer: 7. (b)
Solution:
- Key Idea
In a cyclic thermodynamic process work done
In the given cyclic process, work done
From graph, given






