Heat and Thermodynamics 5 Question 33
34. Match the following for the given process
(2006, 6M)
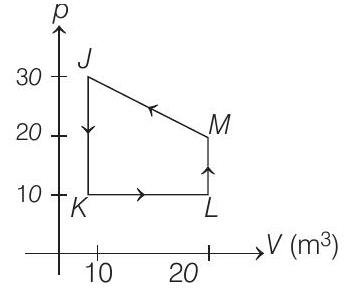
| Column I | Column II | ||
|---|---|---|---|
| (A) | Process $J \rightarrow K$ | (p) | $Q>0$ |
| (B) | Process $K \rightarrow L$ | (q) | $W<0$ |
| (C) | Process $L \rightarrow M$ | (r) | $W>0$ |
| (D) | Process $M \rightarrow J$ | (s) | $Q<0$ |
Show Answer
Answer:
Correct Answer: 34. (A) $\rightarrow$s; (B) $\rightarrow p, r$; (C) $p$; (D) $\rightarrow q, s$
Solution:
- In process $\boldsymbol{J} \rightarrow \boldsymbol{K} V$ is constant whereas $p$ is decreasing. Therefore, $T$ should also decrease.
$\therefore \quad W=0, \Delta U=-$ ve and $Q<0$
In process $\boldsymbol{K} \rightarrow \boldsymbol{L} p$ is constant while $V$ is increasing. Therefore, temperature should also increase.
$\therefore \quad W>0, \Delta U>0$ and $Q>0$
In process $\boldsymbol{L} \rightarrow \boldsymbol{M}$ This is inverse of process $J \rightarrow K$.
$\therefore \quad W=0, \Delta U>0$ and $Q>0$
In process $\boldsymbol{M} \rightarrow \boldsymbol{J}$
$V$ is decreasing. Therefore, $W<0$
$$ \begin{array}{rlrl} & & (p V) _J & <(p V) _M \\ & \therefore & T _J & <T _M \\ \text { or } & \Delta U & <0 \end{array} $$
Therefore, $\quad Q<0$.






