States of Matter - Result Question 97
####1. Points I, II and III in the following plot respectively correspond to (
(2019 Main, 10 April II)
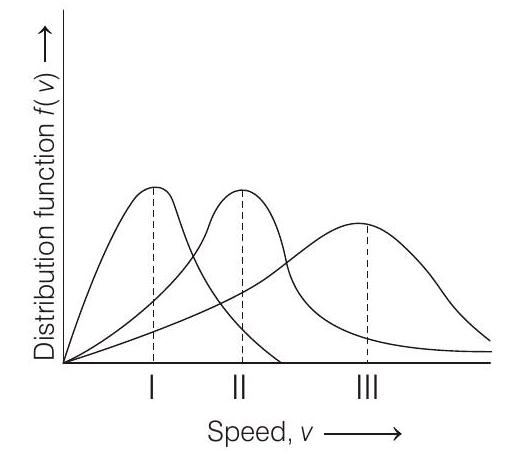
(a)
(b)
(c)
(d)
Show Answer
Answer:
Correct Answer: 1. (d)
Solution:
Key Idea From kinetic gas equation,
Most probable velocity
where,
| Gas | |||
| 2 | 300 | ||
| 28 | 300 | ||
| 32 | 400 |
So,
I. corresponds to
II. corresponds to
III. corresponds to






