Electrochemistry - Result Question 79
####27. For the electrochemical cell, $\operatorname{Mg}(s)\left|Mg^{2+}(a q, 1 M) | Cu^{2+}(a q, 1 M)\right| Cu(s)$
The standard emf of the cell is $2.70 V$ at $300 K$. When the concentration of $Mg^{2+}$ is changed to $x M$, the cell potential changes to $2.67 V$ at $300 K$. The value of $x$ is (Given, $\frac{F}{R}=11500 K V^{-1}$, where $F$ is the Faraday constant and $R$ is the gas contant, $\operatorname{In}(10)=2.30)$
(2018 Adv.)
Show Answer
Answer:
Correct Answer: 27. $(1.4085 M)$
)
Solution:
- (10) Equation of cell reaction according to the cell notation given, is
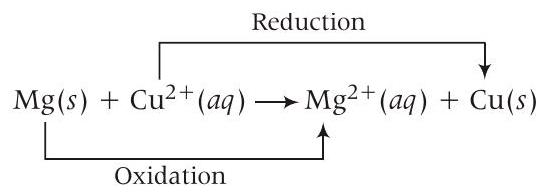
Given, $E _{\text {cell }}^{\circ}=2.70 V, T=300 K$
with $\quad\left[Mg^{2+}(a q)\right]=1 M$ and $\left[Cu^{2+}(a q)\right]=1 M$
and $n=2$
Further, $\quad E _{\text {cell }}=2.67 V$
with $\quad\left[Cu^{2+}(a q)\right]=1 M$
and $\quad\left[Mg^{2+}(a q)\right]=x M$
and $\quad \frac{F}{R}=11500 KV^{-1}$
where $F=$ Faraday constant, $R=$ gas constant From the formula,
$$ E _{\text {cell }}=E _{\text {cell }}^{\circ}-\frac{R T}{n F} \ln \frac{\left[Mg^{2+}(a q)\right]}{\left[Cu^{2+}(a q)\right]} $$
After putting the given values
$$ \begin{aligned}






