Chemistry 12
- Unit 1 The Solid State-Deleted
- Unit 2 Solutions
- Unit 3 Electrochemistry
- Unit 4 Chemical Kinetics
- Unit 5 Surface Chemistry-Deleted
- Unit 6 General Principles And Processes Of Isolation Of Elements-Deleted
- Unit 7 The P Block Elements
- Unit 8 The D And F Block Elements
- Unit 9 Coordination Compounds
- Unit 10 Haloalkanes And Haloarenes
- Unit 11 Alcohols, Phenols And Ethers
- Unit 12 Aldehydes, Ketones And Carboxylic Acids
- Unit 13 Amines
- Unit 14 Biomolecules
- Unit 15 Polymers-Deleted
- Unit 16 Chemistry In Everyday Life-Delelted
Unit 8 The D And F Block Elements
The
There are mainly four series of the transition metals,
Originally the name transition metals was derived from the fact that their chemical properties were transitional between those of
The presence of partly filled d or f orbitals in their atoms makes transition elements different from that of the non-transition elements. Hence, transition elements and their compounds are studied separately. However, the usual theory of valence as applicable to the nontransition elements can be applied successfully to the transition elements also.
Various precious metals such as silver, gold and platinum and industrially important metals like iron, copper and titanium belong to the transition metals series.
In this Unit, we shall first deal with the electronic configuration, occurrence and general characteristics of transition elements with special emphasis on the trends in the properties of the first row (3d) transition metals along with the preparation and properties of some important compounds. This will be followed by consideration of certain general aspects such as electronic configurations, oxidation states and chemical reactivity of the inner transition metals.
8.1 Position in the Periodic Table
The
8.2 Electronic Configurations of the d-Block Elements
In general the electronic configuration of outer orbitals of these elements is


The electronic configurations of outer orbitals of
The
The transition metals and their compounds also exhibit catalytic property and paramagnetic behaviour. All these characteristics have been discussed in detail later in this Unit.
There are greater similarities in the properties of the transition elements of a horizontal row in contrast to the non-transition elements. However, some group similarities also exist. We shall first study the general characteristics and their trends in the horizontal rows (particularly
Intext Question
8.1 Silver atom has completely filled
Answer
Ag has a completely filled
8.3 General Properties of the Transition Elements (d-Block)
We will discuss the properties of elements of first transition series only in the following sections.
8.3.1 Physical Properties
Nearly all the transition elements display typical metallic properties such as high tensile strength, ductility, malleability, high thermal and electrical conductivity and metallic lustre. With the exceptions of

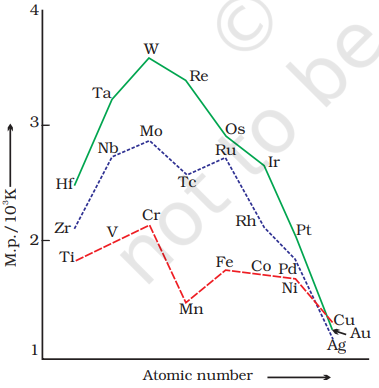
The transition metals (with the exception of
Another generalisation that may be drawn from Fig. 8.2 is that the metals of the second and third series have greater enthalpies of atomisation than the corresponding elements of the first series; this is an important factor in accounting for the occurrence of much more frequent metal - metal bonding in compounds of the heavy transition metals.
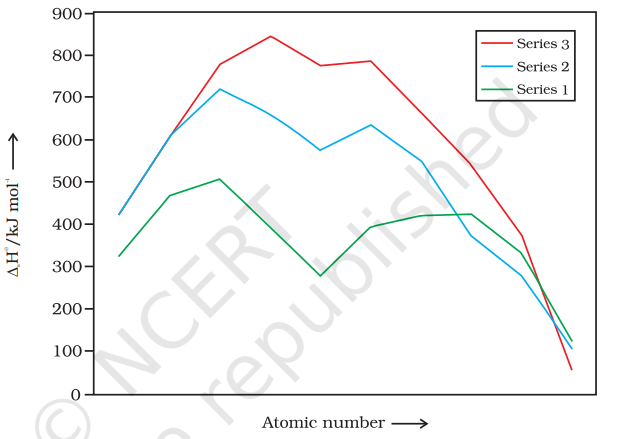
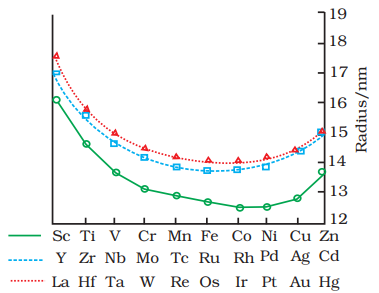
8.3.2 Variation in Atomic and Ionic Sizes of Transition Metals
In general, ions of the same charge in a given series show progressive decrease in radius with increasing atomic number. This is because the new electron enters a
The factor responsible for the lanthanoid contraction is somewhat similar to that observed in an ordinary transition series and is attributed to similar cause, i.e., the imperfect shielding of one electron by another in the same set of orbitals. However, the shielding of one
The decrease in metallic radius coupled with increase in atomic mass results in a general increase in the density of these elements. Thus, from titanium

Example 8.2 Why do the transition elements exhibit higher enthalpies of atomisation?
Solution Because of large number of unpaired electrons in their atoms they have stronger interatomic interaction and hence stronger bonding between atoms resulting in higher enthalpies of atomisation.
Intext Question
8.2 In the series
Answer
The extent of metallic bonding an element undergoes decides the enthalpy of atomization. The more extensive the metallic bonding of an element, the more will be its enthalpy of atomization. In all transition metals (except Zn, electronic configuration:
8.3.3 Ionisation Enthalpies
There is an increase in ionisation enthalpy along each series of the transition elements from left to right due to an increase in nuclear charge which accompanies the filling of the inner d orbitals. Table 8.2 gives the values of the first three ionisation enthalpies of the first series of transition elements. These values show that the successive enthalpies of these elements do not increase as steeply as in the case of non-transition elements. The variation in ionisation enthalpy along a series of transition elements is much less in comparison to the variation along a period of non-transition elements. The first ionisation enthalpy, in general, increases, but the magnitude of the increase in the second and third ionisation enthalpies for the successive elements, is much higher along a series.
The irregular trend in the first ionisation enthalpy of the metals of
The interpretation of variation in ionisation enthalpy for an electronic configuration
The three terms responsible for the value of ionisation enthalpy are attraction of each electron towards nucleus, repulsion between the electrons and the exchange energy. Exchange energy is responsible for the stabilisation of energy state. Exchange energy is approximately proportional to the total number of possible pairs of parallel spins in the degenerate orbitals. When several electrons occupy a set of degenerate orbitals, the lowest energy state corresponds to the maximum possible extent of single occupation of orbital and parallel spins (Hunds rule). The loss of exchange energy increases the stability. As the stability increases, the ionisation becomes more difficult. There is no loss of exchange energy at
The lowest common oxidation state of these metals is +2 . To form the
Although ionisation enthalpies give some guidance concerning the relative stabilities of oxidation states, this problem is very complex and not amenable to ready generalisation.
8.3.4 Oxidation States
One of the notable features of a transition elements is the great variety of oxidation states these may show in their compounds. Table 8.3 lists the common oxidation states of the first row transition elements.

The elements which give the greatest number of oxidation states occur in or near the middle of the series. Manganese, for example, exhibits all the oxidation states from +2 to +7 . The lesser number of oxidation states at the extreme ends stems from either too few electrons to lose or share (
The variability of oxidation states, a characteristic of transition elements, arises out of incomplete filling of
An interesting feature in the variability of oxidation states of the
Low oxidation states are found when a complex compound has ligands capable of
Example 8.3 Name a transition element which does not exhibit variable oxidation states.
Solution
Scandium
Intext Question
8.3 Which of the
Answer
Mn has the maximum number of unpaired electrons present in the
8.3.5 Trends in the M2+/M Standard Electrode Potentials
Table 8.4 contains the thermochemical parameters related to the transformation of the solid metal atoms to
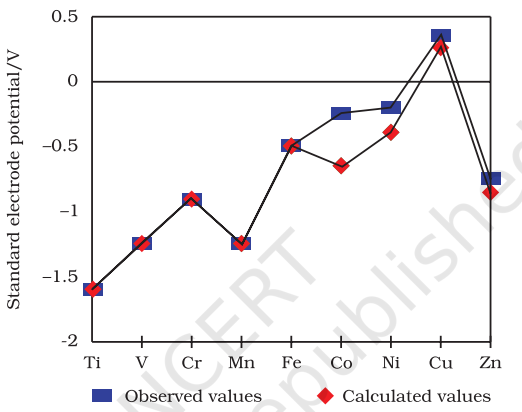
The unique behaviour of

Example 8.4
Why is
Solution
The stability of the half-filled
8.3.6 Trends in the M3+/M2+ Standard Electrode Potentials
An examination of the
8.3.7 Trends in Stability of Higher Oxidation States
Table 8.5 shows the stable halides of the
Although

and the same applies to
However, many copper (I) compounds are unstable in aqueous solution and undergo disproportionation.
The stability of
The ability of oxygen to stabilise the highest oxidation state is demonstrated in the oxides. The highest oxidation number in the oxides (Table 8.6) coincides with the group number and is attained in

Example 8.5
How would you account for the increasing oxidising power in the series
Solution This is due to the increasing stability of the lower species to which they are reduced.
Intext Question
8.5 How would you account for the irregular variation of ionisation enthalpies (first and second) in the first series of the transition elements?
Answer
Ionization enthalpies are found to increase in the given series due to a continuous filling of the inner
In case of first ionization energy,
Second ionization energies are higher than the first since it becomes difficult to remove an electron when an electron has already been taken out. Also, elements like
8.3.8 Chemical Reactivity and E V Values
Transition metals vary widely in their chemical reactivity. Many of them are sufficiently electropositive to dissolve in mineral acids, although a few are ‘noble’—that is, they are unaffected by single acids.
The metals of the first series with the exception of copper are relatively more reactive and are oxidised by
An examination of the
Example 8.6 For the first row transition metals the
| -1.18 | -0.91 | -1.18 | -0.44 | -0.28 | -0.25 | +0.34 |
Explain the irregularity in the above values.
Solution The
Example 8.7 Why is the
Solution
Much larger third ionisation energy of Mn (where the required change is
Intext Questions
8.6 Why is the highest oxidation state of a metal exhibited in its oxide or fluoride only?
Answer
Both oxide and fluoride ions are highly electronegative and have a very small size. Due to these properties, they are able to oxidize the metal to its highest oxidation state.
8.7 Which is a stronger reducing agent
Answer
The following reactions are involved when

8.3.9 Magnetic Properties
When a magnetic field is applied to substances, mainly two types of magnetic behaviour are observed: diamagnetism and paramagnetism (Unit 1). Diamagnetic substances are repelled by the applied field while the paramagnetic substances are attracted. Substances which are attracted very strongly are said to be ferromagnetic. In fact, ferromagnetism is an extreme form of paramagnetism. Many of the transition metal ions are paramagnetic.
Paramagnetism arises from the presence of unpaired electrons, each such electron having a magnetic moment associated with its spin angular momentum and orbital angular momentum. For the compounds of the first series of transition metals, the contribution of the orbital angular momentum is effectively quenched and hence is of no significance. For these, the magnetic moment is determined by the number of unpaired electrons and is calculated by using the ‘spin-only’ formula, i.e.,
where
The magnetic moment increases with the increasing number of unpaired electrons. Thus, the observed magnetic moment gives a useful indication about the number of unpaired electrons present in the atom, molecule or ion. The magnetic moments calculated from the ‘spin-only’ formula and those derived experimentally for some ions of the first row transition elements are given in Table 8.7. The experimental data are mainly for hydrated ions in solution or in the solid state.

Intext Question
8.8 Calculate the ‘spin only’ magnetic moment of
Number of unpaired electrons
Given:
Atomic number
The valence electronic configuration of cobalt (
Hence, valence electronic configuration becomes
Now, Number of unpaired electrons
Spin only magnetic moment
We know that
where
Putting
we get,
8.3.10 Formation of Coloured Ions
When an electron from a lower energy d orbital is excited to a higher energy d orbital, the energy of excitation corresponds to the frequency of light absorbed (Unit 9). This frequency generally lies in the visible region. The colour observed corresponds to the complementary colour of the light absorbed. The frequency of the light absorbed is determined by the nature of the ligand. In aqueous solutions where water molecules are the ligands, the colours of the ions observed are listed in Table 8.8. A few coloured solutions of d–block elements are illustrated in Fig. 8.5.


8.3.11 Formation of Complex Compounds
Complex compounds are those in which the metal ions bind a number of anions or neutral molecules giving complex species with characteristic properties. A few examples are:
8.3.12 Catalytic Properties
The transition metals and their compounds are known for their catalytic activity. This activity is ascribed to their ability to adopt multiple oxidation states and to form complexes. Vanadium(V) oxide (in Contact Process), finely divided iron (in Haber’s Process), and nickel (in Catalytic Hydrogenation) are some of the examples. Catalysts at a solid surface involve the formation of bonds between reactant molecules and atoms of the surface of the catalyst (first row transition metals utilise 3d and 4s electrons for bonding). This has the effect of increasing the concentration of the reactants at the catalyst surface and also weakening of the bonds in the reacting molecules (the activation energy is lowering). Also because the transition metal ions can change their oxidation states, they become more effective as catalysts. For example, iron(III) catalyses the reaction between iodide and persulphate ions.
An explanation of this catalytic action can be given as:
8.3.13 Formation of Interstitial Compounds
Interstitial compounds are those which are formed when small atoms like
(ii) They are very hard, some borides approach diamond in hardness.
(iii) They retain metallic conductivity.
(iv) They are chemically inert.
8.3.14 Alloy Formation
An alloy is a blend of metals prepared by mixing the components. Alloys may be homogeneous solid solutions in which the atoms of one metal are distributed randomly among the atoms of the other. Such alloys are formed by atoms with metallic radii that are within about 15 percent of each other. Because of similar radii and other characteristics of transition metals, alloys are readily formed by these metals. The alloys so formed are hard and have often high melting points. The best known are ferrous alloys: chromium, vanadium, tungsten, molybdenum and manganese are used for the production of a variety of steels and stainless steel. Alloys of transition metals with non transition metals such as brass (copper-zinc) and bronze (copper-tin), are also of considerable industrial importance.
Example 8.9 What is meant by ‘disproportionation’ of an oxidation state? Give an example.
Solution When a particular oxidation state becomes less stable relative to other oxidation states, one lower, one higher, it is said to undergo disproportionation. For example, manganese (VI) becomes unstable relative to manganese(VII) and manganese (IV) in acidic solution.
Intext Question
8.9 Explain why
Answer
In an aqueous medium,
8.4 Some Important Compounds of Transition Elements
8.4.1 Oxides and Oxoanions of Metals
These oxides are generally formed by the reaction of metals with oxygen at high temperatures. All the metals except scandium form MO oxides which are ionic. The highest oxidation number in the oxides, coincides with the group number and is attained in
As the oxidation number of a metal increases, ionic character decreases. In the case of
Thus,
Potassium dichromate is a very important chemical used in leather industry and as an oxidant for preparation of many azo compounds. Dichromates are generally prepared from chromate, which in turn are obtained by the fusion of chromite ore
The yellow solution of sodium chromate is filtered and acidified with sulphuric acid to give a solution from which orange sodium dichromate,
Sodium dichromate is more soluble than potassium dichromate. The latter is therefore, prepared by treating the solution of sodium dichromate with potassium chloride.
Orange crystals of potassium dichromate crystallise out. The chromates and dichromates are interconvertible in aqueous solution depending upon
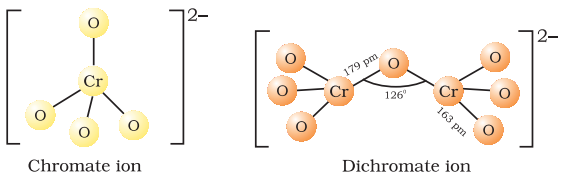
The structures of chromate ion,
Sodium and potassium dichromates are strong oxidising agents; the sodium salt has a greater solubility in water and is extensively used as an oxidising agent in organic chemistry. Potassium dichromate is used as a primary standard in volumetric analysis. In acidic solution, its oxidising action can be represented as follows:
Thus, acidified potassium dichromate will oxidise iodides to iodine, sulphides to sulphur, tin(II) to tin(IV) and iron(II) salts to iron(III). The half-reactions are noted below:
The full ionic equation may be obtained by adding the half-reaction for potassium dichromate to the half-reaction for the reducing agent, for e.g.,
Potassium permanganate
Potassium permanganate is prepared by fusion of
Commercially it is prepared by the alkaline oxidative fusion of
In the laboratory, a manganese (II) ion salt is oxidised by peroxodisulphate to permanganate.
Potassium permanganate forms dark purple (almost black) crystals which are isostructural with those of
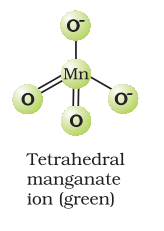
It has two physical properties of considerable interest: its intense colour and its diamagnetism along with temperature-dependent weak paramagnetism. These can be explained by the use of molecular orbital theory which is beyond the present scope.
The manganate and permanganate ions are tetrahedral; the
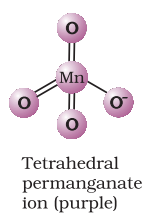
Acidified permanganate solution oxidises oxalates to carbon dioxide, iron(II) to iron(III), nitrites to nitrates and iodides to free iodine. The half-reactions of reductants are:
The full reaction can be written by adding the half-reaction for
If we represent the reduction of permanganate to manganate, manganese dioxide and manganese(II) salt by half-reactions,
We can very well see that the hydrogen ion concentration of the solution plays an important part in influencing the reaction. Although many reactions can be understood by consideration of redox potential, kinetics of the reaction is also an important factor. Permanganate at
A few important oxidising reactions of
1. In acid solutions:
(a) Iodine is liberated from potassium iodide :
(b)
(c) Oxalate ion or oxalic acid is oxidised at
(d) Hydrogen sulphide is oxidised, sulphur being precipitated:
(e) Sulphurous acid or sulphite is oxidised to a sulphate or sulphuric acid:
(f) Nitrite is oxidised to nitrate:
2. In neutral or faintly alkaline solutions:
(a) A notable reaction is the oxidation of iodide to iodate:
(b) Thiosulphate is oxidised almost quantitatively to sulphate:
(c) Manganous salt is oxidised to
Note: Permanganate titrations in presence of hydrochloric acid are unsatisfactory since hydrochloric acid is oxidised to chlorine.
Uses: Besides its use in analytical chemistry, potassium permanganate is used as a favourite oxidant in preparative organic chemistry. Its uses for the bleaching of wool, cotton, silk and other textile fibres and for the decolourisation of oils are also dependent on its strong oxidising power.
THE INNER TRANSITION ELEMENTS ( f-BLOCK)
The f-block consists of the two series, lanthanoids (the fourteen elements following lanthanum) and actinoids (the fourteen elements following actinium). Because lanthanum closely resembles the lanthanoids, it is usually included in any discussion of the lanthanoids for which the general symbol Ln is often used. Similarly, a discussion of the actinoids includes actinium besides the fourteen elements constituting the series. The lanthanoids resemble one another more closely than do the members of ordinary transition elements in any series. They have only one stable oxidation state and their chemistry provides an excellent opportunity to examine the effect of small changes in size and nuclear charge along a series of otherwise similar elements. The chemistry of the actinoids is, on the other hand, much more complicated. The complication arises partly owing to the occurrence of a wide range of oxidation states in these elements and partly because their radioactivity creates special problems in their study; the two series will be considered separately here.
8.5 The Lanthanoids
The names, symbols, electronic configurations of atomic and some ionic states and atomic and ionic radii of lanthanum and lanthanoids (for which the general symbol Ln is used) are given in Table 8.9.
8.5.1 Electronic Configurations
It may be noted that atoms of these elements have electronic configuration with
8.5.2 Atomic and Ionic Sizes
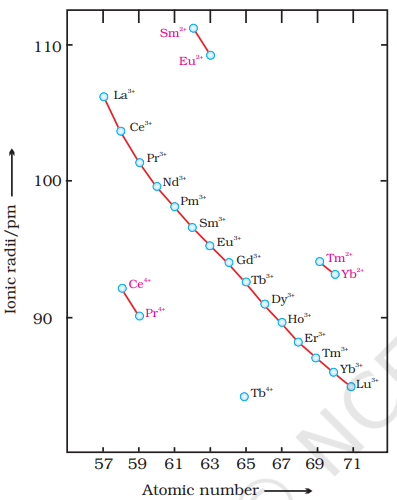
The overall decrease in atomic and ionic radii from lanthanum to lutetium (the lanthanoid contraction) is a unique feature in the chemistry of the lanthanoids. It has far reaching consequences in the chemistry of the third transition series of the elements. The decrease in atomic radii (derived from the structures of metals) is not quite regular as it is regular in
The cumulative effect of the contraction of the lanthanoid series, known as lanthanoid contraction, causes the radii of the members of the third transition series to be very similar to those of the corresponding members of the second series. The almost identical radii of
8.5.3 Oxidation States
In the lanthanoids,

8.5.4 General Characteristics
All the lanthanoids are silvery white soft metals and tarnish rapidly in air. The hardness increases with increasing atomic number, samarium being steel hard. Their melting points range between 1000 to
Many trivalent lanthanoid ions are coloured both in the solid state and in aqueous solutions. Colour of these ions may be attributed to the presence of
The first ionisation enthalpies of the lanthanoids are around
In their chemical behaviour, in general, the earlier members of the series are quite reactive similar to calcium but, with increasing atomic number, they behave more like aluminium. Values for
are in the range of –2.2 to –2.4 V except for Eu for which the value is - 2.0 V. This is, of course, a small variation. The metals combine with hydrogen when gently heated in the gas. The carbides, Ln3C, Ln2C3 and LnC2 are formed when the metals are heated with carbon. They liberate hydrogen from dilute acids and burn in halogens to form halides. They form oxides M2O3 and hydroxides M(OH)3. The hydroxides are definite compounds, not just hydrated oxides. They are basic like alkaline earth metal oxides and hydroxides. Their general reactions are depicted in Fig. 8.7.
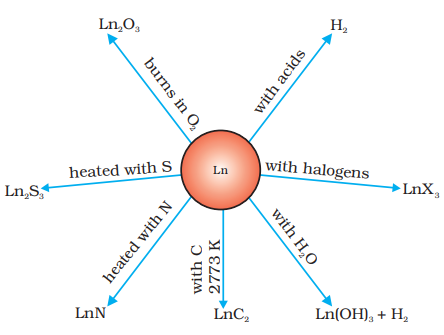
The best single use of the lanthanoids is for the production of alloy steels for plates and pipes. A well known alloy is mischmetall which consists of a lanthanoid metal (~ 95%) and iron (~ 5%) and traces of S, C, Ca and Al. A good deal of mischmetall is used in Mg-based alloy to produce bullets, shell and lighter flint. Mixed oxides of lanthanoids are employed as catalysts in petroleum cracking. Some individual Ln oxides are used as phosphors in television screens and similar fluorescing surfaces.
8.6 The Actinoids

The actinoids are radioactive elements and the earlier members have relatively long half-lives, the latter ones have half-life values ranging from a day to 3 minutes for lawrencium
8.6.1 Electronic Configurations
All the actinoids are believed to have the electronic configuration of
8.6.2 Ionic Sizes
The general trend in lanthanoids is observable in the actinoids as well. There is a gradual decrease in the size of atoms or
8.6.3 Oxidation States
There is a greater range of oxidation states, which is in part attributed to the fact that the
The actinoids show in general +3 oxidation state. The elements, in the first half of the series frequently exhibit higher oxidation states. For example, the maximum oxidation state increases from +4 in

8.6.4 General Characteristics and Comparison with Lanthanoids
The actinoid metals are all silvery in appearance but display a variety of structures. The structural variability is obtained due to irregularities in metallic radii which are far greater than in lanthanoids.
The actinoids are highly reactive metals, especially when finely divided. The action of boiling water on them, for example, gives a mixture of oxide and hydride and combination with most non metals takes place at moderate temperatures. Hydrochloric acid attacks all metals but most are slightly affected by nitric acid owing to the formation of protective oxide layers; alkalies have no action.
The magnetic properties of the actinoids are more complex than those of the lanthanoids. Although the variation in the magnetic susceptibility of the actinoids with the number of unpaired 5 f electrons is roughly parallel to the corresponding results for the lanthanoids, the latter have higher values.
It is evident from the behaviour of the actinoids that the ionisation enthalpies of the early actinoids, though not accurately known, but are lower than for the early lanthanoids. This is quite reasonable since it is to be expected that when 5f orbitals are beginning to be occupied, they will penetrate less into the inner core of electrons. The 5f electrons, will therefore, be more effectively shielded from the nuclear charge than the 4f electrons of the corresponding lanthanoids. Because the outer electrons are less firmly held, they are available for bonding in the actinoids.
A comparison of the actinoids with the lanthanoids, with respect to different characteristics as discussed above, reveals that behaviour similar to that of the lanthanoids is not evident until the second half of the actinoid series. However, even the early actinoids resemble the lanthanoids in showing close similarities with each other and in gradual variation in properties which do not entail change in oxidation state. The lanthanoid and actinoid contractions, have extended effects on the sizes, and therefore, the properties of the elements succeeding them in their respective periods. The lanthanoid contraction is more important because the chemistry of elements succeeding the actinoids are much less known at the present time.
Intext Question
8.10 Actinoid contraction is greater from element to element than lanthanoid contraction. Why?
Answer
In actinoids,
8.7 Some Applications of d- and f-Block Elements
Iron and steels are the most important construction materials. Their production is based on the reduction of iron oxides, the removal of impurities and the addition of carbon and alloying metals such as
Summary
The
Corresponding to the filling of
Successive ionisation enthalpies do not increase as steeply as in the main group elements with increasing atomic number. Hence, the loss of variable number of electrons from
The transition elements vary widely in their chemical behaviour. Many of them are sufficiently electropositive to dissolve in mineral acids, although a few are ’noble’. Of the first series, with the exception of copper, all the metals are relatively reactive.
The transition metals react with a number of non-metals like oxygen, nitrogen, sulphur and halogens to form binary compounds. The first series transition metal oxides are generally formed from the reaction of metals with oxygen at high temperatures. These oxides dissolve in acids and bases to form oxometallic salts. Potassium dichromate and potassium permanganate are common examples. Potassium dichromate is prepared from the chromite ore by fusion with alkali in presence of air and acidifying the extract. Pyrolusite ore
The two series of inner transition elements, lanthanoids and actinoids constitute the
There are many useful applications of the
Exercises
8.1 Write down the electronic configuration of:
(i)
(ii)
(iii)
(iv)
(v)
(vi)
(vii)
(viii)
Answer
(i)
Or,
(ii)
Or,
(iii)
Or,
(iv)
Or,
(v)
Or,
(vi)
Or,
(vii)
8.2 Why are
Answer
Electronic configuration of
Electronic configuration of
It is known that half-filled and fully-filled orbitals are more stable. Therefore,
8.3 Explain briefly how +2 state becomes more and more stable in the first half of the first row transition elements with increasing atomic number?
Answer
Element (+2 state)
Electronic configuration
In all the elements listed, the removal of two
8.4 To what extent do the electronic configurations decide the stability of oxidation states in the first series of the transition elements? Illustrate your answer with examples.
Answer
The elements in the first-half of the transition series exhibit many oxidation states with Mn exhibiting maximum number of oxidation states ( +2 to +7 ). The stability of +2 oxidation state increases with the increase in atomic number. This happens as more electrons are getting filled in the
8.5 What may be the stable oxidation state of the transition element with the
following
Answer
| Electronic configuration in ground state | Stable oxidation states | |
|---|---|---|
| (i) | ||
| (ii) | ||
| (iii) | ||
| (iv) | ||
| (v) | There is no |
8.6 Name the oxometal anions of the first series of the transition metals in which the metal exhibits the oxidation state equal to its group number.
Answer
8.7 What is lanthanoid contraction? What are the consequences of lanthanoid contraction?
Answer
As we move along the lanthanoid series, the atomic number increases gradually by one. This means that the number of electrons and protons present in an atom also increases by one. As electrons are being added to the same shell, the effective nuclear charge increases. This happens because the increase in nuclear attraction due to the addition of proton is more pronounced than the increase in the interelectronic repulsions due to the addition of electron. Also, with the increase in atomic number, the number of electrons in the 4 forbital also increases. The 4 felectrons have poor shielding effect. Therefore, the effective nuclear charge experienced by the outer electrons increases. Consequently, the attraction of the nucleus for the outermost electrons increases. This results in a steady decrease in the size of lanthanoids with the increase in the atomic number. This is termed as lanthanoid contraction.
Consequences of lanthanoid contraction
(i) There is similarity in the properties of second and third transition series.
ii. Separation of lanthanoids is possible due to lanthanide contraction.
(iii) It is due to lanthanide contraction that there is variation in the basic strength of lanthanide hydroxides. (Basic strength decreases from
8.8 What are the characteristics of the transition elements and why are they called transition elements? Which of the
Answer
Transition elements are those elements in which the atoms or ions (in stable oxidation state) contain partially filled
Elements such as
8.9 In what way is the electronic configuration of the transition elements different from that of the non-transition elements?
Answer
Transition metals have a partially filled
The non-transition elements either do not have a
8.10 What are the different oxidation states exhibited by the lanthanoids?
Answer
In the lanthanide series, +3 oxidation state is most common i.e., Ln(III) compounds are predominant. However, +2 and +4 oxidation states can also be found in the solution or in solid compounds.
8.11 Explain giving reasons:
(i) Transition metals and many of their compounds show paramagnetic behaviour.
(ii) The enthalpies of atomisation of the transition metals are high.
(iii) The transition metals generally form coloured compounds.
(iv) Transition metals and their many compounds act as good catalyst.
Answer
(i) Transition metals show paramagnetic behaviour. Paramagnetism arises due to the presence of unpaired electrons with each electron having a magnetic moment associated with its spin angular momentum and orbital angular momentum. However, in the first transition series, the orbital angular momentum is quenched. Therefore, the resulting paramagnetism is only because of the unpaired electron.
(ii) Transition elements have high effective nuclear charge and a large number of valence electrons. Therefore, they form very strong metallic bonds. As a result, the enthalpy of atomization of transition metals is high.
(iii) Most of the complexes of transition metals are coloured. This is because of the absorption of radiation from visible light region to promote an electron from one of the
(iv) The catalytic activity of the transition elements can be explained by two basic facts.
(a) Owing to their ability to show variable oxidation states and form complexes, transition metals form unstable intermediate compounds. Thus, they provide a new path with lower activation energy,
(b) Transition metals also provide a suitable surface for the reactions to occur.
8.12 What are interstitial compounds? Why are such compounds well known for transition metals?
Answer
Transition metals are large in size and contain lots of interstitial sites. Transition elements can trap atoms of other elements (that have small atomic size), such as H, C, N, in the interstitial sites of their crystal lattices. The resulting compounds are called interstitial compounds.
8.13 How is the variability in oxidation states of transition metals different from that of the non-transition metals? Illustrate with examples.
Answer
In transition elements, the oxidation state can vary from +1 to the highest oxidation state by removing all its valence electrons. Also, in transition elements, the oxidation states differ by
8.14 Describe the preparation of potassium dichromate from iron chromite ore. What is the effect of increasing
Answer
(i) Preparation of
(a) Potassium dichromate is obtained by the fusion of chromite ore
(b) The solution is filtered and treated with sulphuric acid.
(c) Now sodium dichromate is treated with potassium chloride.
As a result, potassium dichromate is produced.
(ii) Effect of increasing
On increasing
8.15 Describe the oxidising action of potassium dichromate and write the ionicequations for its reaction with:
(i) iodide (ii) iron(II) solution and (iii)
Answer
(i)
(ii)
(iii)
8.16 Describe the preparation of potassium permanganate. How does the acidifiedpermanganate solution react with (i) iron(II) ions (ii)
Answer
Potassium permanganate can be prepared from pyrolusite
The green mass can be extracted with water and then oxidized either electrolytically or by passing chlorine/ozone into the solution.
Electrolytic oxidation
At anode, manganate ions are oxidized to permanganate ions.
Oxidation by chlorine
Oxidation by ozone
(i) Acidified
(ii) Acidified potassium permanganate oxidizes
(iii) Acidified potassium permanganate oxidizes oxalic acid to carbon dioxide.
8.17 For
Use this data to comment upon:
(i) The stability of
(ii) The ease with which iron can be oxidised as compared to a similar process for either chromium or manganese metal.
Answer
(i) The
(ii) The reduction potentials for the given pairs increase in the following order.
So, the oxidation of
8.18 Predict which of the following will be coloured in aqueous solution?
Answer
Only the ions that have electrons in
| Element | Atomic Number | Ionic State | Electronic configuration in ionic state |
|---|---|---|---|
| 22 | |||
| 23 | |||
| 29 | |||
| 21 | |||
| 25 | |||
| 26 | |||
| 27 |
From the above table, it can be easily observed that only
8.19 Compare the stability of +2 oxidation state for the elements of the first transition series.
Answer
From the above table, it is evident that the maximum number of oxidation states is shown by
8.20 Compare the chemistry of actinoids with that of the lanthanoids with specialreference to:
(i) electronic configuration (iii) oxidation state
(ii) atomic and ionic sizes and (iv) chemical reactivity.
Answer
(i) Electronic configuration
The general electronic configuration for lanthanoids is
(ii) Oxidation states
The principal oxidation state of lanthanoids is (+3). However, sometimes we also encounter oxidation states of +2 and +4 . This is because of extra stability of fully-filled and half-filled orbitals. Actinoids exhibit a greater range of oxidation states. This is because the
(iii) Atomic and lonic sizes
Similar to lanthanoids, actinoids also exhibit actinoid contraction (overall decrease in atomic and ionic radii). The contraction is greater due to the poor shielding effect of 5 forbitals.
(iv) Chemical reactivity
In the lanthanide series, the earlier members of the series are more reactive. They have reactivity that is comparable to
8.21 How would you account for the following:
(i) Of the
(ii) Cobalt(II) is stable in aqueous solution but in the presence of complexing reagents it is easily oxidised.
(iii) The
Answer
(i)
(ii)
(iii) The ions in
8.22 What is meant by ‘disproportionation’? Give two examples of disproportionation reaction in aqueous solution.
Answer
It is found that sometimes a relatively less stable oxidation state undergoes an oxidation - reduction reaction in which it is simultaneously oxidised and reduced. This is called disproportionation.
Forexample,
(i)
(ii)
8.23 Which metal in the first series of transition metals exhibits +1 oxidationstate most frequently and why?
Answer
In the first transition series, Cu exhibits +1 oxidation state very frequently. It is because
8.24 Calculate the number of unpaired electrons in the following gaseous ions:
Answer
| Gaseous ions | Number of unpaired electrons | |
|---|---|---|
| (i) | 4 | |
| (ii) | 3 | |
| (iii) | 2 | |
| (vi) | 1 |
8.25 Give examples and suggest reasons for the following features of the transition metal chemistry:
(i)The lowest oxide of transition metal is basic, the highest is amphoteric/acidic.
(ii)A transition metal exhibits highest oxidation state in oxides and fluorides.
(iii) The highest oxidation state is exhibited in oxoanions of a metal.
Answer
(i) In the case of a lower oxide of a transition metal, the metal atom has a low oxidation state. This means that some of the valence electrons of the metal atom are not involved in bonding. As a result, it can donate electrons and behave as a base.
On the other hand, in the case of a higher oxide of a transition metal, the metal atom has a high oxidation state. This means that the valence electrons are involved in bonding and so, they are unavailable. There is also a high effective nuclear charge.
As a result, it can accept electrons and behave as an acid.
For example,
(ii) Oxygen and fluorine act as strong oxidising agents because of their high electronegativities and small sizes. Hence, they bring out the highest oxidation states from the transition metals. In other words, a transition metal exhibits higher oxidation states in oxides and fluorides. For example, in
(iii) Oxygen is a strong oxidising agent due to its high electronegativity and small size. So, oxo-anions of a metal have the highest oxidation state. For example, in
8.26 Indicate the steps in the preparation of:
(i)
(ii)
Answer
(i)
Potassium dichromate
Step (1):Preparation of sodium chromate
Step (2):Conversion of sodium chromate into sodium dichromate
Step(3):Conversion of sodium dichromate to potassium dichromate
Potassium chloride being less soluble than sodium chloride is obtained in the form of orange coloured crystals and can be removed by filtration.
The dichromate ion
Potassium permanganate
The green mass can be extracted with water and then oxidized either electrolytically or by passing chlorine/ozone into the solution.
Electrolytic oxidation
At anode, manganate ions are oxidized to permanganate ions.
Green Purple
Oxidation by chlorine
Oxidation by ozone
8.27 What are alloys? Name an important alloy which contains some of thelanthanoid metals. Mention its uses.
Answer
An alloy is a solid solution of two or more elements in a metallic matrix. It can either be a partial solid solution or a complete solid solution. Alloys are usually found to possess different physical properties than those of the component elements.
An important alloy of lanthanoids is Mischmetal. It contains lanthanoids (94-95
Uses
(1) Mischmetal is used in cigarettes and gas lighters.
(2) It is used in flame throwing tanks.
(3) It is used in tracer bullets and shells.
8.28 What are inner transition elements? Decide which of the following atomic numbers are the atomic numbers of the inner transition elements: 29, 59, 74, 95, 102, 104.
Answer
Inner transition metals are those elements in which the last electron enters the
8.29 The chemistry of the actinoid elements is not so smooth as that of the Lanthanoids. Justify this statement by giving some examples from the oxidation state of these elements.
Answer
Lanthanoids primarily show three oxidation states
8.30 Which is the last element in the series of the actinoids? Write the electronic configuration of this element. Comment on the possible oxidation state of this element.
Answer
The last element in the actinoid series is lawrencium, Lr. Its atomic number is 103 and its electronic configuration is
8.31 Use Hund’s rule to derive the electronic configuration of
Answer
Ce :
Magnetic moment can be calculated as:
Where,
The electronic configuration of
In
8.32 Name the members of the lanthanoid series which exhibit +4 oxidation state and those which exhibit +2 oxidation state. Try to correlate this type of behavior with the electronic configurations of these elements.
Answer
The lanthanides that exhibit +2 and +4 states are shown in the given table. The atomic numbers of the elements are given in the parenthesis.
Ce after forming
Eu after forming
8.33 Compare the chemistry of the actinoids with that of lanthanoids with reference to:
(i) electronic configuration
(ii) oxidation states and
(iii) chemical reactivity.
Answer
Electronic configuration
The general electronic configuration for lanthanoids is
Oxidation states
The principal oxidation state of lanthanoids is (+3). However, sometimes we also encounter oxidation states of +2 and +4 . This is because of extra stability of fully-filled and half-filled orbitals. Actinoids exhibit a greater range of oxidation states. This is because the
Chemical reactivity
In the lanthanide series, the earlier members of the series are more reactive. They have reactivity that is comparable to Ca. With an increase in the atomic number, the lanthanides start behaving similar to Al. Actinoids, on the other hand, are highly reactive metals, especially when they are finely divided. When they are added to boiling water, they give a mixture of oxide and hydride. Actinoids combine with most of the non-metals at moderate temperatures. Alkalies have no action on these actinoids. In case of acids, they are slightly affected by nitric acid (because of the formation of a protective oxide layer).
8.34 Write the electronic configurations of the elements with the atomic numbers 61, 91, 101, and 109.
Answer
| Atomic number | Electronic configuration |
|---|---|
| 61 | |
| 91 | |
| 101 | |
| 109 |
8.35 Compare the general characteristics of the first series of the transition metals with those of the second and third series metals in the respective vertical columns. Give special emphasis on the following points:
(i) electronic configurations,
(ii) oxidation states,
(iii) ionisation enthalpies, and
(iv) atomic sizes.
Answer
(i) In the
We know that elements in the same vertical column generally have similar electronic configurations.
In the first transition series, two elements show unusual electronic configurations:
Similarly, there are exceptions in the second transition series. These are:
There are some exceptions in the third transition series as well. These are:
As a result of these exceptions, it happens many times that the electronic configurations of the elements present in the same group are dissimilar.
(ii) In each of the three transition series the number of oxidation states shown by the elements is the maximum in the middle and the minimum at the extreme ends.
However, +2 and +3 oxidation states are quite stable for all elements present in the first transition series. All metals present in the first transition series form stable compounds in the +2 and +3 oxidation states. The stability of the +2 and +3 oxidation states decreases in the second and the third transition series, wherein higher oxidation states are more important.
For example
(iii) In each of the three transition series, the first ionisation enthalpy increases from left to right. However, there are some exceptions. The first ionisation enthalpies of the third transition series are higher than those of the first and second transition series. This occurs due to the poor shielding effect of 4 felectrons in the third transition series.
Certain elements in the second transition series have higher first ionisation enthalpies than elements corresponding to the same vertical column in the first transition series. There are also elements in the
(iv) Atomic size generally decreases from left to right across a period. Now, among the three transition series, atomic sizes of the elements in the second transition series are greater than those of the elements corresponding to the same vertical column in the first transition series. However, the atomic sizes of the elements in the third transition series are virtually the same as those of the corresponding members in the second transition series. This is due to lanthanoid contraction.
8.36 Write down the number of 3d electrons in each of the following ions:
Indicate how would you expect the five 3d orbitals to be occupied for these hydrated ions (octahedral).
Answer
8.37 Comment on the statement that elements of the first transition series possess many properties different from those of heavier transition elements.
Answer
The properties of the elements of the first transition series differ from those of the heavier transition elements in many ways.
(i) The atomic sizes of the elements of the first transition series are smaller than those of the heavier elements (elements of
However, the atomic sizes of the elements in the third transition series are virtually the same as those of the corresponding members in the second transition series. This is due to lanthanoid contraction.
(ii) +2 and +3 oxidation states are more common for elements in the first transition series, while higher oxidation states are more common for the heavier elements.
(iii) The enthalpies of atomisation of the elements in the first transition series are lower than those of the corresponding elements in the second and third transition series.
(iv) The melting and boiling points of the first transition series are lower than those of the heavier transition elements. This is because of the occurrence of stronger metallic bonding (
(v) The elements of the first transition series form low-spin or high-spin complexes depending upon the strength of the ligand field. However, the heavier transition elements form only low-spin complexes, irrespective of the strength of the ligand field.
8.38 What can be inferred from the magnetic moment values of the following complex species?
Example Magnetic Moment (BM)
Answer
Magnetic moment
For value
For value
For value
For value
For value
(i)
For in transition metals, the magnetic moment is calculated from the spin-only formula. Therefore,
We can see from the above calculation that the given value is closest to
Hence, we can say that
(ii)
We can see from the above calculation that the given value is closest to
Hence, we can say that
(iii)
We can see from the above calculation that the given value is closest to
Hence, we can say that






