Surface Chemistry
Surface Chemistry is the branch of chemistry which deals with the phenomenon that occurs on the surfaces or interfaces, such phenomenon includes corrosion. catalysis, crystallization, etc
Adsorption
PYQ-2023- Surface chemistry-Q5
PYQ-2023- Surface chemistry-Q8
Due to unbalanced attraction forces, accumulation of molecular species at the surface rather than in the bulk of a solid or liquid is termed as adsorption. The molecular species accumulates at the surface is termed as adsorbate and the material on the surface of which the adsorption takes place is called adsorbent, e.g..
(i) $O_2$, $H_2$, $C1_2$ gases are adsorbed on the surface of charcoal.
(ii) Silica gels adsorb water molecules from air. Charcoal, silica gel, metals such as Ni, Cu, Ag, Pt and colloids are some adsorbents.
Important Characteristics of Adsorption
-
It is specific and selective in nature.
-
Adsorption is spontaneous process, therefore change in free energy ($\Delta G$) is negative.
$\Delta G$ = $\Delta H $– $T\Delta S$
For the negative value of $\Delta G$,in a system, in which randomness decreases, $\Delta H$ must be negative. Hence, adsorption is always exothermic.
Adsorption of hydrogen over Pt is called occlusion.
Desorption
It is a process of removing an adsorbed substance from a surface on which it is adsorbed, is known as desorption.
Distinction between Adsorption and Absorption
PYQ-2023- Surface chemistry-Q3
PYQ-2023- Surface chemistry-Q2
| Adsorption | Absorption | |
|---|---|---|
| 1. | It involves unequal distribution of the molecular species in bulk and at the surface. | It involves uniform distribution of the molecular species throughout the bulk. |
| 2. | It is a surface phenomenon. | It occurs throughout the body of material. |
| 3. | It is rapid in the beginning. | It occurs at a uniform rate. |
Sorption
It is a process in which both adsorption and absorption take place simultaneously, the term sorption is simply used.
Positive and Negative Adsorption
PYQ-2023- Surface chemistry-Q3
When the concentration of the adsorbate is more on the surface of the adsorbent than in the bulk, it is called positive adsorption.
On the other hand, if the concentration of the adsorbate is less relative to its concentration in the bulk, it is called negative adsorption, e.g., when a dilute solution of KCl is shaken with blood charcoal, it shows negative adsorption.
Distinction between Physisorption and Chemisorption
PYQ-2023-Chemical-Kinetics-Q11
PYQ-2023- Surface chemistry-Q2
| Physisorption | Chemisorption | |
|---|---|---|
| 1. | It arises when the adsorbate molecules accumulate on the surface of adsorbent on account of weak van der Waals’ forces. | It arises when the adsorbate molecules accumulate on the surface of adsorbent on account of chemical bonds. |
| 2 | It occurs at low temperature. | It occurs at high temperature. |
| 3 | Heat of adsorption is low and it is in the range of $20-40 \mathrm{~kJ} / \mathrm{mol}$. | Heat of adsorption is high and it is in the range of $80-240 \mathrm{~kJ} / \mathrm{mol}$. |
| 4 | It is a reversible process. | It is an irreversible process. |
| 5. | Multilayer adsorption and thus, adsorbed layer is several molecules thick. | Monolayer adsorption. Thus, adsorbed layer is only unimolecular in thickness. |
Factors Affecting Adsorption
(a) Nature of adsorbent same gas may be adsorbed to different extents on different adsorbent.
(b) Surface area of the adsorbent Greater the surface area, greater is the extent of adsorption.
(c) Nature of the gas being adsorbed Greater is the critical temperature of a gas, greater are the van der Waals’ forces of attraction and thus, greater is the adsorption.
(d) Temperature Adsorption is an exothermic process involving the equilibrium :
Gas (adsorbate) + Solid (adsorbent) ⇔ Gas adsorbed on solid + Heat
Applying Le-Chatelier’s principle, increase of temperature decreases the adsorption and vice- versa.
(e) Pressure Adsorption increases with pressure at constant temperature. The effect is large if temperature is kept constant at low value.
(f) Activation of the solid adsorbent Activation means increasing the adsorbing power of the solid adsorbent. This can be done by subdividing the solid adsorbent or by removing the gases already adsorbed by passing superheated steam.
Adsorption Isotherms
It is the plot of the mass of gas adsorbed per gram of adsorbent (x / m) versus equilibrium pressure at constant temperature.
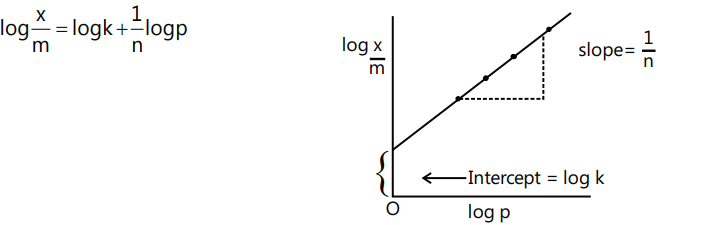
Freundlich Adsorption Isotherm
PYQ-2023- Surface chemistry-Q9
PYQ-2023- Surface chemistry-Q6
It gave an empirical relationship between the quantity of gas adsorbed by unit mass of solid adsorbent and pressure at a particular temperature. It can be expressed by the equation.
$\frac{x}{m} = kp^1/n$ …(i)
Where, x is the mass of the gas adsorbed on mass m of the adsorbent at pressure p, k and n are constants which depend on the nature of the adsorbent and the gas at a particular temperature
(i) At low pressure, the graph is almost straight line which indicates the x/m is directly proportional to the pressure. This may be expressed as: $\frac{x}{m} \propto p$ or $\frac{x}{m}= p$
(ii) At high pressure, the graph becomes almost constant which mean that x/m becomes independent of pressure. This may be expressed as:
$\frac{x}{m} $= Constant or $\frac{x}{m} = p^0$
$p^0$= 1 (pressure raised to the power zero = 1)
(iii) This, in the intermediate range of pressure, x/m will depend upon the power of pressure which lies between 0 and 1 i.e., fractional power of pressure. This may be expressed as
$\frac{x}{m} \propto p^{1 / n} ; \frac{x}{m}=k p^{1 / n}$
Langmuir Adsorption Isotherm
According to Langmuir, the degree of adsorption is directly proportional to e, i.e., the fraction of surface area occupied.
$\frac{x}{m} \propto \theta = k\theta$
$$ \begin{aligned} & \theta=\frac{K_1 P}{K_2+K_1 P} \ & \theta=\frac{\left(K_1 P\right) K_2}{\left(K_2+K_1 P\right) K_2} \ & \theta=\frac{b p}{1+b p}, \text { where } \mathrm{b}=\frac{K_1}{K_2} \end{aligned} $$
The amount of the gas adsorbed on the surface of adsorbent is proportional to $\theta$. $$ \begin{aligned} \frac{x}{m} & =\theta \ \frac{x}{m} & =\mathrm{K}_3 \theta …….(iv) \end{aligned} $$
Substituting the value of equation (iii), we get $$ \frac{x}{m}=\mathrm{K}_3 \frac{b p}{1+b p} $$ $\frac{x}{m}=\frac{a p}{1+b p} \ldots$ (v), where $\mathrm{a}=\mathrm{K}_3 \mathrm{~b}$ is constant.
Thus, Equation (v) is the required Langmuir equation, and a & b are Langmuir constant
Catalysis
Catalyst is a chemical substance which can change the rate of reaction without being used up in that reaction and this process is known as catalysis
| Process | Catalyst |
|---|---|
| 1. Haber’s process of $\mathrm{NH}_3$ | Finely divided $\mathrm{Fe}$ (Mo acts as promoter) |
| 2. Ostwald’s process for manufacture of nitric acid | Platinized asbestos |
| 3. Contact process for $\mathrm{H}_2 \mathrm{SO}_4$ | Platinized asbestos or $\mathrm{V}_2 \mathrm{O}_5$ |
| 4. Lead chamber process for $\mathrm{H}_2 \mathrm{SO}_4$ | Nitric oxide |
| 5. Decon’s process | $\mathrm{CuCl}$ |
A catalyst may be positive (i.e., increases rate of reaction) or negative (i.e., decreases rate of reaction).
Types of Catalysis
(a) Homogeneous catalysis In this catalysis, and the catalyst reactants are in the same physical state [phase], e.g.,
$2SO_2(g) +O_2(g) \xrightarrow {NO_2(g)} 2SO_2(g)$
(b) Heterogeneous catalysis In heterogeneous catalysis, catalyst is present in a different phase than that of reactants, e.g.,
$2KClO_3(s) +O_2(g) \xrightarrow {MnO_2(g)} 2KCl + O_2(g)$
(c) Autocatalysis When one of the product of a reaction acts as catalyst, the process is called autocatalysis.
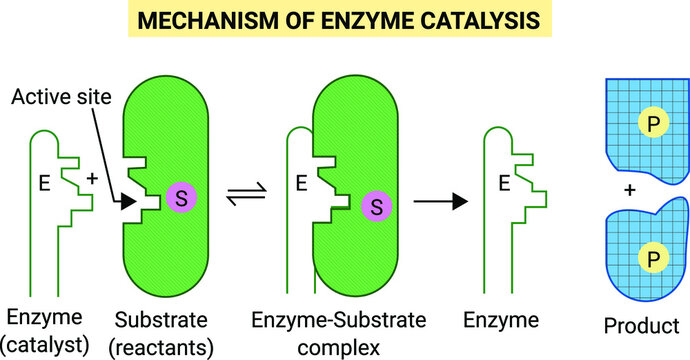
Adsorption Theory of Heterogeneous Catalysis
The mechanism involves five steps:
(i) Diffusion of reactants to the surface of the catalyst
(ii) Adsorption of reactant molecules on the surface of the catalyst.
(iii) Occurrence of chemical reaction on the catalyst’s surface through formation of an intermediate.
(iv) Desorption of reaction products from t he catalyst surface.
(v) Diffusion of reaction products away from the catalyst’s surface
Important Features of Solid Catalysts
(i) Activity The activity of a catalyst depends upon the strength of chemisorption to a large extent. The adsorption should be reasonably strong but not so strong that they become immobile and no space is available for other reactants to get adsorbed.
(ii) Selectivity The selectivity of a catalyst is its ability to direct a reaction to yield a particular product, e.g., starting with Hz and CO using different catalysts, we get different products.
Shape–selective catalysis The catalytic reaction that depends upon the pore structure of the catalyst and the size of the reactant and product molecules is called shape-selective catalysis
Cracking Isomerization of hydrocarbons in the presence of zeolites is an example of shape-selective catalysis.
An important zeolite catalyst used in the petroleum industry is ZSM-S.It converts alcohols directly into gasoline.
Enzyme Catalysis
Enzymes are complex nitrogenous organic compounds which are Produced by living plants and animals. They are actually protein molecules of high molecular mass and form colloidal solutions in water.
They are also known as biochemical catalysis.
Mechanism of Enzyme Catalysis
Characteristics of Enzyme Catalysis
-
High efficiency: One molecule of an enzyme may transform one million molecule of reactant per minute.
-
Highly specific nature Each enzyme catalyst cannot catalyse more than one reaction.
-
Optimum temperature: Enzyme catalyst gives higher yield at optimum temperature i.e., at 298-310 K. Human body temperature, i.e., at being 310 K is suited for enzyme catalysed reactions.
-
Optimum pH: The rate of an enzyme catalysed reaction is maximum at optimum pH range 5 to 7.
-
Activators: Activators like ions such as $Na^+ ,Ca^{2+}, Mn^{2+}$ help in the activation of enzymes which cannot act on their own strength.
-
Co-enzyme: Co-enzymes are the substance having nature similar to the enzyme and their presence increases the enzyme activity. Mostly vitamins act as co-enzymes.
-
Effect of Inhibitors: Inhibitors slow down the rate of enzymatic reaction. The use of many drugs is based on enzyme inhibition action of those drugs in the bod
Electrophoresis
The phenomenon has found applications in:
(i) Determining nature of charge on sol particles.
(ii) Determining electrokinetic potential.
(iii) Coagulation
Colloidal Solution
PYQ-2023- Surface chemistry-Q1
The foundation of colloid chemistry was laid by an English scientist, Thomas Graham, in 1861.
Table: Comparison of suspensions, colloids, and true solutions.
| S.No. | Property | Suspension | Colloid | True Solution or Crystalloid |
|---|---|---|---|---|
| (i) | Particle size | $>10^{-5} \mathrm{~cm}$ or $10^3 \mathrm{~A}^{\circ}$ or $100 \mathrm{~m} \mu$ | $10^{-7}$ to $10^{-5} \mathrm{~cm}$ or $10 \mathrm{~A}^{\circ}$ to $10^3\mathrm{~A}^{\circ}$ or $1 \mathrm{~m} \mu$ to $100 \mathrm{~mm}$ | $<10^{-7} \mathrm{~cm}$ or $10 \mathrm{~A}^{\circ}$ or $1 \mathrm{~m} \mu$ |
| (ii) | Visibility | Visible with naked eye | Visible with ultramicroscope | Not visible with any of the optical means |
| (iii) | Seperation | (a) with filter paper | Possible | Not possible |
| (b) with membranes | Possible | Not possible | ||
| (iv) | Diffusion | Does not diffuse | Diffuse very slowly | Diffuses rapidly |
| (v) | Settling | Settles under gravity | Does not settle but it may settle under Centrifuge | Does not settle |
| (vi) | Nature | Heterogeneous | Heterogeneous | Homogeneous |
| (vii) | Tyndal effect Brownian moment | May or may not show | Clear shows | Does not show |
Types of Colloidal Solutions
A colloidal system is made of two phases. The substance distributed as the colloidal particles is called the dispersed phase, the internal phase, or the discontinuous phase. The second continuous phase in which the colloidal particles are dispersed is called the dispersion medium. For example, for a colloidal solution of copper in water, copper particles constitute the dispersed phase and water the dispersion medium.
Depending on the physical states of the dispersed phase or dispersion medium, colloidal solutions are of eight types:
| Type of Colloidal Solution | Dispersed Phase | Dispersion Medium | Example |
|---|---|---|---|
| Foam | Gas | Liquid | Whipped Cream, Shaving Cream, Soda Water |
| Solid Foam | Gas | Solid | Cork, Pumice Stone, Foam Rubber |
| Liquid Aerosol | Liquid | Gas | Fog, Mist, Clouds |
| Emulsion | Liquid | Liquid | Milk, Hair Cream |
| Gel | Liquid | Solid | Butter, Cheese Curd, Jellies, Boot Polish |
| Smoke (Aerosol) | Solid | Gas | Dust, Soot in Air |
| Sol (Liquid) | Solid | Liquid | Ink, Colloidal Gold |
| Solid Sol | Solid | Solid | Ruby Glass (Gold Dispersed in Glass), Alloys |
A colloidal dispersion of one gas in another is not possible since the two gases would give a homogeneous molecular structure.
Properties of Colloidal Solutions
-
Heterogeneous - Colloidal particles in a solution differ in sizes and are not homogeneously distributed throughout the solution.
-
Visibility - Colloidal particles cannot be seen with the naked eye or with the help of a microscope. It is a well-known fact that no particle is visible if its diameter is less than half the wavelength of light used. The visible light has a greater wavelength than the size of colloidal particles.
-
Filterability - Colloidal particles pass through an ordinary filter paper but do not pass through parchment and other fine membranes.
-
Surface Tension and Viscosity
For Lyophobic sols, surface tension and viscosity are not very different from those of the medium, as there is very slight interaction between the suspended particles and the medium. On the other hand, Lyophilic sols show a high degree of solvation of the particles, and therefore, the properties of the medium are modified. Thus, the viscosity is much higher for the sol than for the medium. Furthermore, the surface tension of the sol is lower than that of the pure medium.
-
Colour - The colour of a hydrophobic sol depends on the wavelength of the light scattered by the dispersed particles. The wavelength of the scattered light again depends on the size and the nature of particles. For example, the colour of silver sol changes with the particle (suspended) diameter in solution.
- Colour of Ag sol: Orange-yellow, orange, red, purple, violet
- Particle diameter:
- $6 \times 10^{-5}$ mm
- $9 \times 10^{-5}$ mm
- $13 \times 10^{-5}$ mm
- $15 \times 10^{-5}$ mm
-
Colligative Properties - These properties depend on the number of solute particles in the solution. In the case of colloidal solutions, colloidal particles are aggregates of many ions or smaller molecules, and when compared to true solutions or normal solutions, the total number of particles of solute in the solution are very less, and hence these solutions exhibit colligative properties to a lesser extent.
Coagulation
PYQ-2023- Surface chemistry-Q4
When an electrolyte is added to colloidal solution, the particles of the solution take up oppositively charged ions and thus, get neutralized. The neutral particles then comes closer and get accumulated to form bigger particles which settle down. Hence coagulations is defined as a process which involves precipitation of a colloidal solution by addition of excess of electrolyte.
Isoelectric Point
The point at which the colloidal particles do not carry charge and possess minimum stability
Distribution of Charge
The potential difference set-up across the surface of separation of two oppositely charged layers just in contact with each other is known as electro kinetic potential or zeta potential.
Surfactants
PYQ-2023- Surface chemistry-Q7
- Surfactants are a class of chemical compounds used to reduce the surface tension (or interfacial tension) between two or more compounds, such as two liquids, a gas and a liquid, or a liquid and a solid.
- Surfactants are amphiphilic organic molecules that are classified as organic compounds.
- It comprises both hydrophobic and hydrophilic groups, which is what it basically signifies. A surfactant, in other words, has both a water-insoluble and a water-soluble component.
- Surfactants have the ability to diffuse in water and adsorb at air-water interfaces, which is one of their most prevalent features. It can also adsorb at the oil-water interface, where oil is combined with water. The insoluble group in water can move out of the bulk water phase and into the air or oil phase. The water-soluble head group, on the other hand, frequently remains in the water phase.
Mode of Action
When a large enough number of surfactant molecules are added to a solution, they begin to combine. In the bulk aqueous phase, they form micelles, which are structures or aggregates. The surfactant heads (hydrophilic heads) remain exposed to water or the surrounding liquid as the micelle forms. The tails (hydrophilic heads) converge at the structure’s centre, where they are shielded from water. Different types of aggregates can be generated, such as spherical or cylindrical micelles or lipid bilayers.
Types of surfactants
Surfactants are divided into numerous categories based on their polar head group
Anionic Surfactants
The surfactant is called anionic if the charge on the head group (hydrophilic end) is negative. It has anionic functional groups, including sulphate, sulfonate, phosphate, and carboxylates at its head. Sulfates, sulfonates, and gluconates are examples of anionic surfactants.
Cationic Surfactants
Similarly, cationic means the head group (hydrophilic end) has a positive charge. Cationic surfactants include alkyl ammonium chlorides, which are commonly used.
Zwitterionic Surfactants
On their hydrophilic end, zwitterionic surfactants, also known as amphoteric surfactants, have both positive and negative charges. On the same molecule, they have both cationic and anionic centres. It basically has a net charge of zero. Surfactants of this kind include betaines and amino oxides.
Non-Ionic Surfactants
Non-ionic surfactants are typically neutral, and their hydrophilic end has no charge. Covalently bound oxygen-containing hydrophilic groups are bonded to hydrophobic parent structures in non-ionic surfactants. They can be used to emulsify oils and appear to remove organic soils more effectively than anionic surfactants. Non-ionic surfactants are less susceptible to water hardness and produce less foam than anionic surfactants. Ethoxylates, alkoxylates, and cocamide are examples of non-ionic surfactants.






