Oxidation Reaction
Points to remember in Oxidation Reaction
$\mathrm{KMnO}_{4}$ (in both medium) or
$K_2 Cr_2 O_7$ (in acidic medium)
Aldehyde $\longrightarrow$ Acid
$1^{\circ}$ Alcohol $\longrightarrow$ Acid
$2^{\circ}$ Alcohol $\longrightarrow$ Ketone
$3^{\circ}$ Alcohol $\longrightarrow$ No reaction
PCC (Pyridinium chlorochromate)
$CrO_{3} /HCl / Pyridine$
$1^{\circ} ROH \longrightarrow$ Aldehyde
$2^{\circ} ROH \longrightarrow$ Ketone
$3^{\circ} ROH \longrightarrow$ No reaction
Cu/573 K
$1^{\circ}$ Alcohol $\longrightarrow$ Aldehyde
$2^{\circ}$ Alcohol $\longrightarrow$ Ketone
$3^{\circ}$ Alcohol $\longrightarrow$ Alkene
$HIO_{4}$ (Periodic Acid):
Condition : Vicinal diol, $\alpha$ - Hydroxy ketone & $\alpha$-diketone can oxidize by $HIO_{4}$
Baeyer’s reagent and $ OsO_{4}+ NaHSO_{3}$

Baeyer-Villiger oxidation (m-CPBA or $CH_{3} CO_{3} H$)

Priority of shift (O accepting aptitude)
$R^{\prime}$ = $Ph > $ Ethyl > Methyl
Prilezhaev reaction
oxidation by $\mathrm{HNO}_{3}$
Aldehyde $\longrightarrow$ Acid
$1^{\circ}$ Alcohol $\longrightarrow$ Acid
$2^{\circ}$ Alcohol $\longrightarrow$ no reaction
$3^{\circ}$ Alcohol $\longrightarrow$ No reaction
oxidation by $\mathrm{MnO}_{2}$
$1^{\circ}$ Alcohol $\longrightarrow$ Aldehyde
$2^{\circ}$ Alcohol $\longrightarrow$ Ketone
$3^{\circ}$ Alcohol $\longrightarrow$ No reaction
Note : Only allylic and benzylic alcohols are oxidized by $MnO_{2}$.
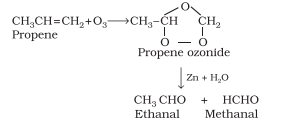
Hydroboration Oxidation reaction
PYQ-2024-Hydrocarbons-Q16, PYQ-2024-Hydrocarbons-Q8,
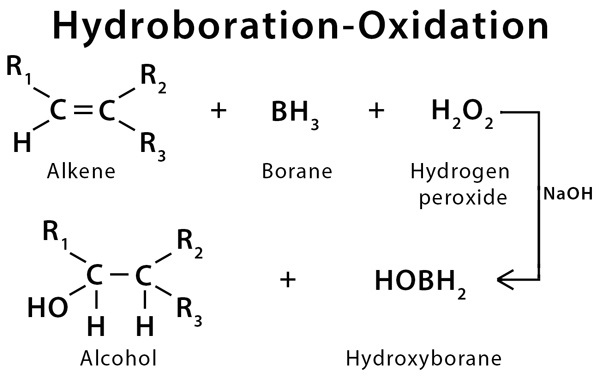
oxymercuration demercuration reaction
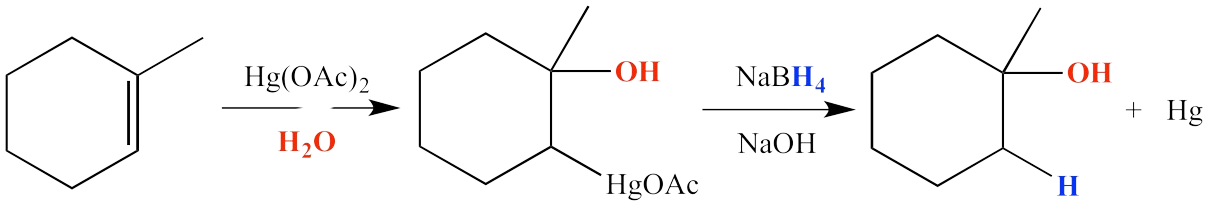
Ozonolysis