Chemistry In Everyday Life
DRUGS
The classification of drugs is done on various grounds to highlight their functional areas, throwing light on their mechanism.
Drugs: Drugs are chemicals of low molecular masses (~100 – 500u), interacting with macromolecular targets to produce a biological response.
Medicines: Medicines are chemicals, useful in diagnosis, prevention, and treatment of diseases. A dose consumed higher than the recommended can cause harm.
Therapeutic Effect: It is a desirable or beneficial drug effect like treatment of symptoms and cure of a disease on a living body. Such use of chemicals for therapeutic effect is called chemotherapy.
Classification Of Drugs
PYQ-2023 Chemistry in Everyday Life Q2
| On the basis of | Explanation |
|---|---|
| Pharmacological Effect | This effect is used by the specialists to prescribe a drug for a particular disorder from a whole category of drugs. E.g. Analgesics kill pain while antiseptics kill or arrest a microorganism’s growth. |
| E.g. Analgesics kill pain while antiseptics kill or arrest a microorganism’s growth. | |
| Drug action | Release of a specific compound in a body or a biochemical process under consideration can be treated by various ways by a single category of drug. |
| E.g., Antihistamine inhibits the action of the compound, histamine, which causes inflammation in the body. Thus, various ways exist to block the action of histamines. | |
| Chemical structure | This typically depends on the chemical structure of the drug. Similar functional groups show common pharmacological activity. |
| E.g., Structure of sulphonamide show the common functional group | |
| Molecular targets | Drugs are very specific in action and have their targets decided. They interact with biomolecules such as carbohydrates, lipids, proteins, and nucleic acids. Common structural features show the same mechanism of action. |
Drug Target Interaction
Enzymes as drug targets
(a) Competitive Inhibition: Competitive Inhibitors are the drugs which attach themselves (as shown in the Fig. 32.2) onto the active site of an enzyme by competing with the substrate for the space.
(b) Non-Competitive Inhibition: Non-competitive drugs change the shape of the active site by binding themselves to an allosteric site, due to which the substrate is unable to identify the active site and is thus disabled to attach itself. The presence of a strong covalent bond between an enzyme and an inhibitor blocks the enzyme. Degradation of this complex gives a new enzyme. Non-competitive inhibitor changes the active site of the enzyme after binding at allosteric site.
Receptors as drug targets
Antagonists are the drugs which bind with the receptor site and inhibit its natural function. These are useful when blocking of message is required. Drugs that mimic the natural messenger by switching on the receptor are called agonists. These are useful when there is a lack of natural chemical messenger.
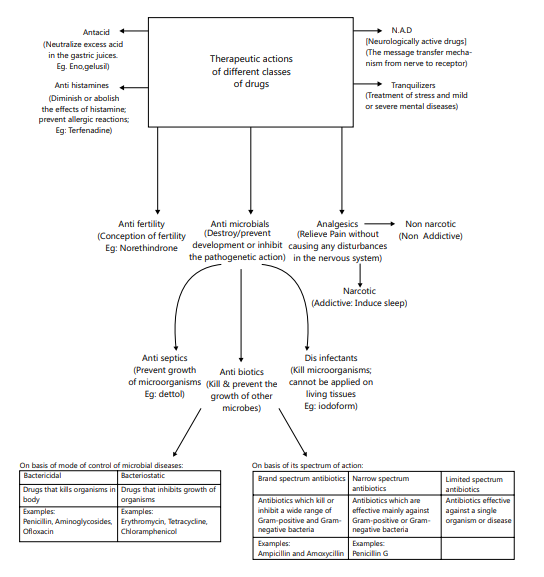
Therapeutic Actions Of Different Classes Of Drugs
PYQ-2023 Chemistry in Everyday Life Q1, PYQ-2023 Chemistry in Everyday Life Q4, PYQ-2023 Chemistry in Everyday Life Q5, PYQ-2023 Chemistry in Everyday Life Q6, PYQ-2023 Chemistry in Everyday Life Q7, PYQ-2023 Chemistry in Everyday Life Q9, PYQ-2023 Chemistry in Everyday Life Q10, PYQ-2023 Chemistry in Everyday Life Q11
CHEMICALS IN FOOD / FOOD ADDITIVES
PYQ-2023 Chemistry In Everyday Life Q8
| No. | Name of food additive | Examples |
|---|---|---|
| 1 | Artificial Sweetening Agents | Aspartame, Sucralose and Alitame |
| 2 | Food preservatives | Sugar, Salts, Sodium benzoate |
| Food colors | Allura Red AC, Tartrazine | |
| 3 | Nutritional supplements | Vitamins, minerals etc. |
| 4 | Fat emulsifiers and stabilizing agents | Egg yolk (where the main emulsifying chemical is Lecithin) |
| 5 | Antioxidants | Butylated hydroxytoluene (BHT), Butylated hydroxyanisole (BHA) |
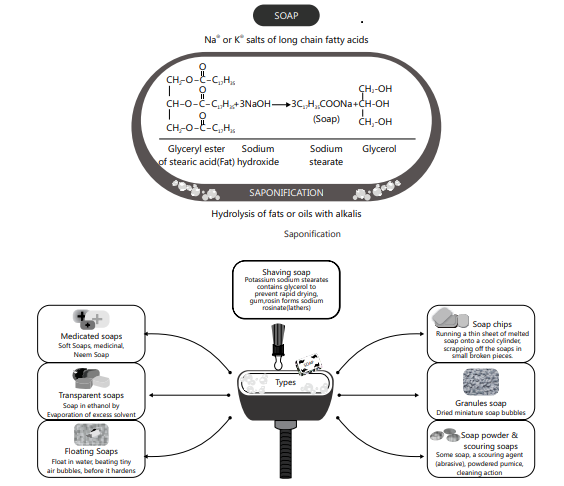
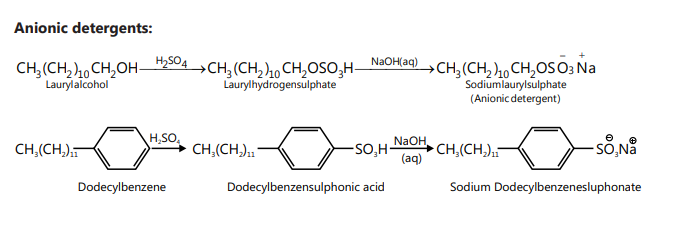
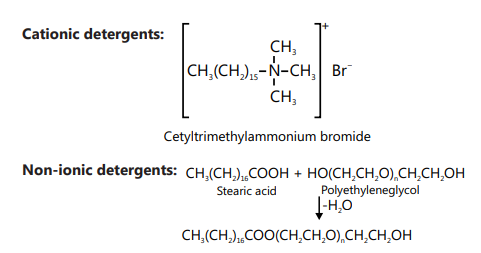
SOAPS AND DETERGENTS
Neurotransmitter
PYQ-2023 Chemistry in Everyday Life Q1
Noradrenaline is both a neurotransmitter and a hormone. As a neurotransmitter, it’s a chemical messenger that helps transmit nerve signals across nerve endings to another nerve cell, muscle cell, or gland cell.
Environmental Chemistry
Environmental Pollution : Process of contamination of the environment with harmful wastes arising mainly from human activities.
Acid Rain:
PYQ-2023 Environmental Chemistry Q7
SO2, nitrogen oxides & acidic soots. Sulphurdioxide & nitrogen dioxide interact with water vapours in presence of sunlight to form sulphuric acid & nitric acid units.
Green House Effect & Global Warming:
PYQ-2023 Environmental Chemistry Q9
The green house gases (CO2, CH4, O3, CFC’S ) in the atmosphere form a thick cover around the earth. About 75% of the solar energy reaching the earth is absorbed by the earth surface. The IR radiations coming from sun are not absorbed by atmospheric gases but Earth absorbs these IR radiations of short wavelength. As a result of this the temperature of earth stands rising. Eventually, earth starts emitting infrared radiations of longer wavelengths. The partially radiated infrared radiations from the earth are absorbed by the greenhouse gases. This results in excessive heating of Earth’s atmosphere. Thus the greenhouse gases add to the heating of atmosphere. This causes global warming. The atmosphere traps the sun’s heat near earth’s surface and keeps it warm. The reemission of the earth’s energy absorbed by CO2 and other greenhouse gases present near the earths surface and its radiation back to the earth is called green house effect.
Photochemical smog :
PYQ-2023 Environmental Chemistry Q3, PYQ-2023 Environmental Chemistry Q5, PYQ-2023 Environmental Chemistry Q8
Photochemical smog, also known as summer smog, is a type of smog that is produced when UV light originating from the sun interacts with the oxides of nitrogen present in the atmosphere. This type of smog usually manifests as a brown haze and is most commonly seen in highly populated cities that are placed in relatively warm climates.
Water Pollution:
PYQ-2023 Environmental Chemistry Q4, PYQ-2023 Environmental Chemistry Q6
Biochemical Oxygen Demand (BOD) : The measure of the total contamination caused by compounds which can be oxidised in the presence of microorganisms. The BOD is taken as a realistic measure of water quality – clean water would have a BOD value of less than 5 ppm whereas highly polluted river water could have a BOD value of 17 ppm or more.
Land Pollution :
Caused by pesticides and other chemicals which are added to the soil to grow better crops.
Insecticides are the pesticides used to Control of insects by insecticides helps to curb disease and protect crops. Organo chlorines are a group of compounds which have been developed and used as insecticides.Examples: DDT (dichlorodiphenyl trichloro ethane) organo chlorines are stable in the environment, toxic to insects in small amounts, but much less go to humans, and because they are organic compounds not very soluble in water. The advantage of these insecticides is that, bring persistent
Fungicides are the pesticides used to check the growth of fungi. Fungi, are plants without chlorophyll, they cannot use solar energy for preparing their food. They live as saprophytes on decaying organic matter or as parasites at the expense of living organisms. Hence they are considered to be a threat to human interests
Freons :
The Freon gas is a colourless, odourless, noninflammable, noncorrosive gas of low toxicity introduced as refrigerants in the 1930s. They also proved helpful as propellants for aerosols and in numerous technical applications. Freon (trademark) comprises several simple fluorinated aliphatic organic compounds utilized for commercial and industrial purposes. Apart from fluorine and carbon, Freons often include hydrogen, bromine, or chlorine.






