Simple Harmonic Motion 4 Question 2
2. A massless spring
(2019 Main, 9 April II)
(a)
(b)
(c)
(d)
Show Answer
Answer:
Correct Answer: 2. (d)
Solution:
- The given situation is shown in the figure given below
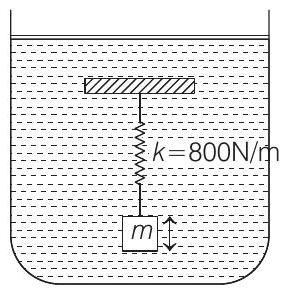
When vibrations of mass are suddenly stopped, oscillation energy (or stored energy of spring) is dissipated as heat, causing rise of temperature.
So, conversation of energy gives
where,
and
Substituting values given in question, we have






