Modern Physics 7 Question 48
54. A neutron of kinetic energy
(a) Find the allowed values of the energy of the neutron and that of the atom after the collision.
(b) If the atom gets de-excited subsequently by emitting radiation, find the frequencies of the emitted radiation.
[Given : Mass of He atom
Ionization energy of
Show Answer
Answer:
Correct Answer: 54. (a)
(b)
Solution:
- (a) Let
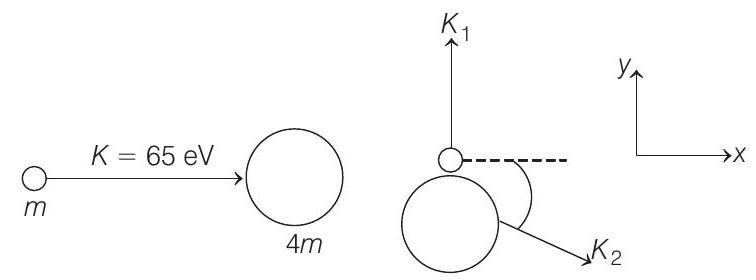
From conservation of linear momentum along
Similarly, applying conservation of linear momentum in
Squaring and adding Eqs. (i) and (ii), we get
Now, during collision, electron can be excited to any higher energy state. Applying conservation of energy, we get
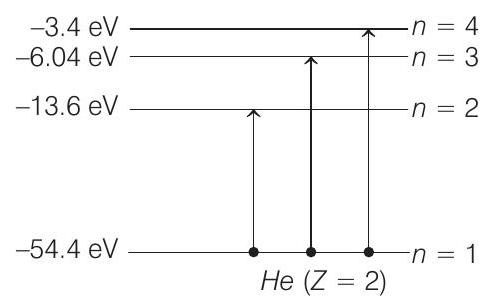
or
Substituting in (v), we get
Solving (iv) and (vi), we get
Similarly, when we put
Put in Eq. (v), we get
Solving Eqs. (iv) and (vii), we get
Similarly, when we put
Put in Eq. (v), we get
Now, solving Eqs. (iv) and (viii), we get
But since the kinetic energy cannot have the negative values, the electron will not jump to third excited state or
Therefore, the allowed values of
(b) Possible emission lines are only three as shown in figure. The corresponding frequencies are
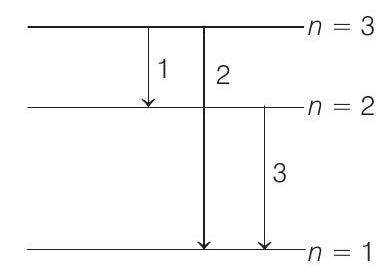
Hence, the frequencies of emitted radiations are






