Modern Physics 1 Question 17
14. The wavelength of the first spectral line in the Balmer series of hydrogen atom is
(2011)
(a)
(b)
(c)
(d)
Show Answer
Solution:
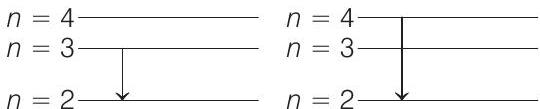
For hydrogen or hydrogen type atoms,
In the transition from
Substituting the values, we have






