Heat and Thermodynamics 5 Question 23
24. Starting with the same initial conditions, an ideal gas expands from volume
(a)
(b)
(c)
(d)
Show Answer
Solution:
- The corresponding
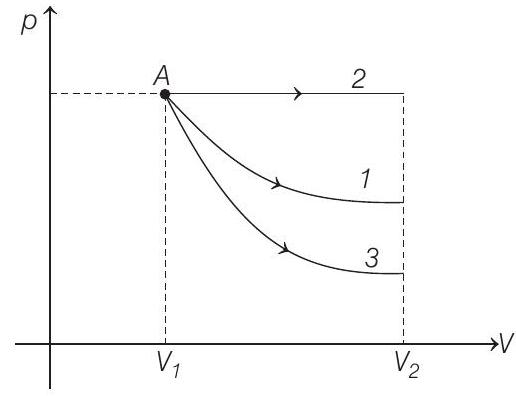
Area under the graph gives the work done by the gas.






